Abstract
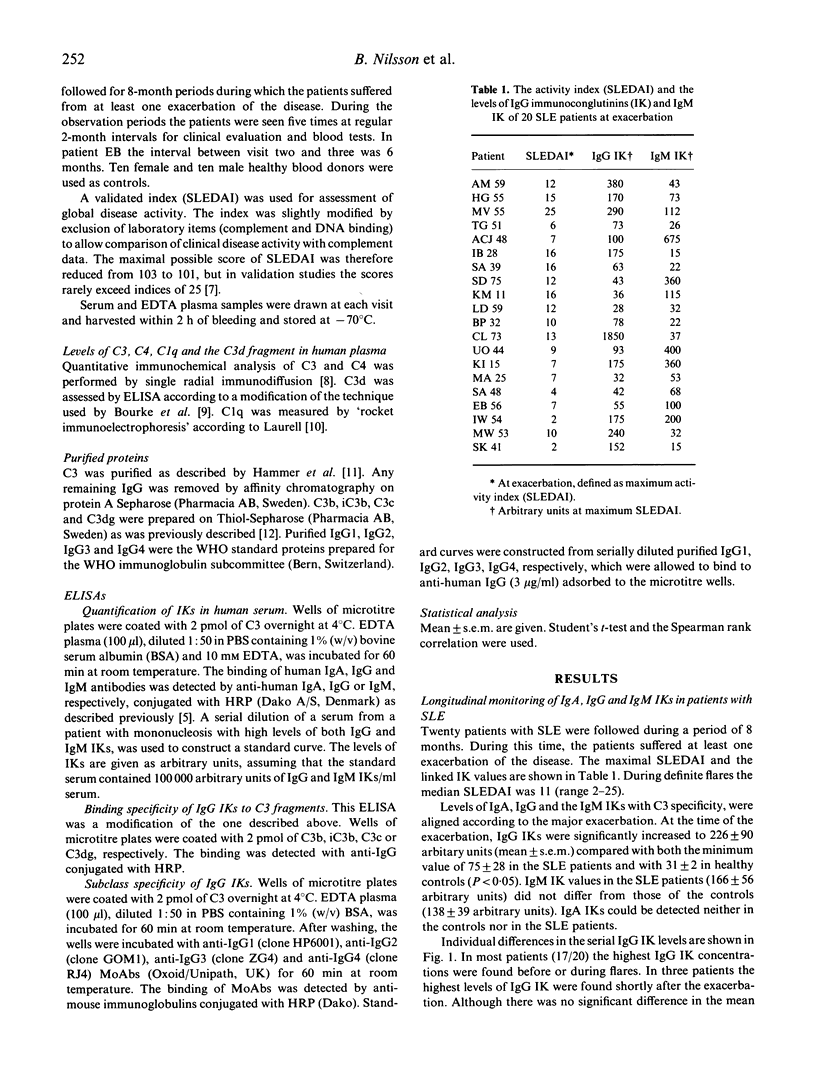
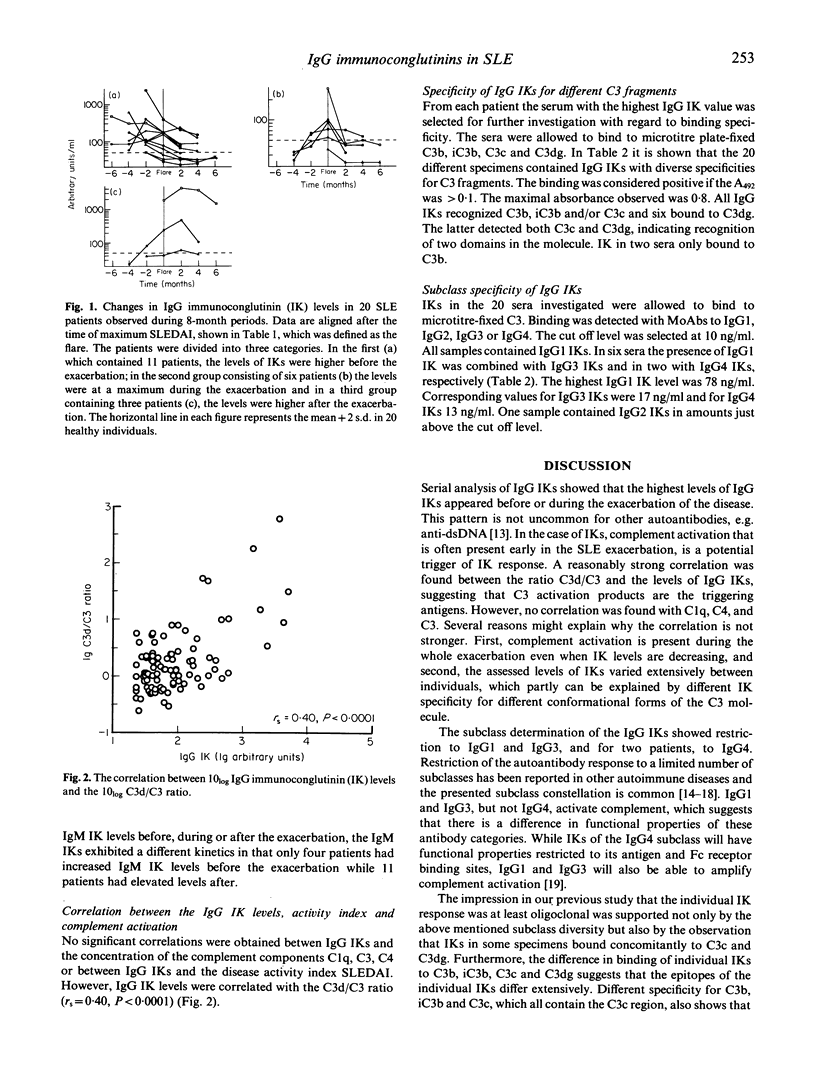
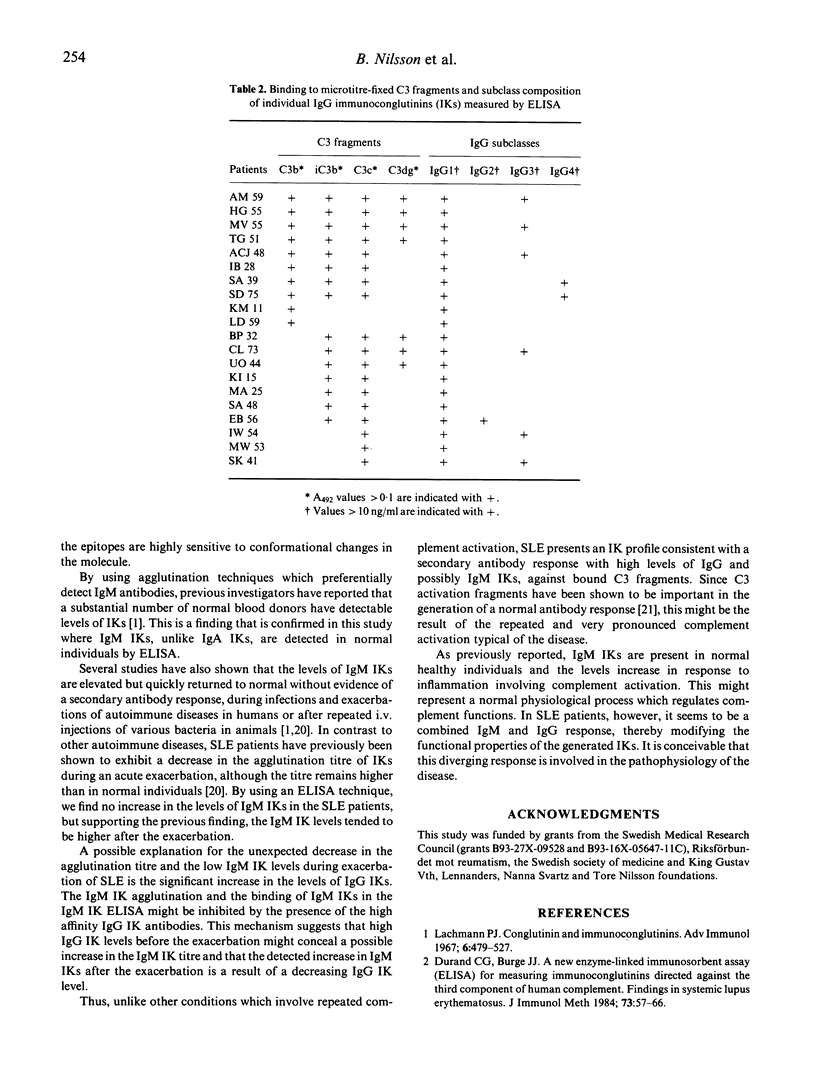
The levels of IgA, IgG and IgM immunoconglutinins (IK) were assessed in sera from 20 patients with SLE which were followed for 8-month periods. At the time of the exacerbation, IgG IKs were significantly increased to 226 +/- 90 arbitrary units (mean +/- s.e.m.) compared with both the minimum value of 75 +/- 28 in the SLE patients and with 31 +/- 2 in healthy controls (P < 0.05). There was no difference between SLE patients and controls in the levels of IgM and IgA IKs. Most of the SLE patients in this material showed maximal IgG IK levels before exacerbation, but there was no correlation between the clinical disease index and the levels of IgG IK. The specificity of IgG IKs showed a broad diversity for microtitre-fixed C3b, iC3b, C3c and C3dg. The antibodies were of IgG1, IgG3 and in two patients, IgG4 subclass. IgG IKs were correlated to the C3d/C3 ratio which suggested that the IK responses were secondary to C3 activation. In summary, unlike other conditions associated with complement activation where elevated IgM IKs are common, an increase in IgG IK levels was observed. It is possible that this diverging IK response contributes to the pathophysiology of the disease.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernard P., Prost C., Aucouturier P., Durepaire N., Denis F., Bonnetblanc J. M. The subclass distribution of IgG autoantibodies in cicatricial pemphigoid and epidermolysis bullosa acquisita. J Invest Dermatol. 1991 Aug;97(2):259–263. doi: 10.1111/1523-1747.ep12480369. [DOI] [PubMed] [Google Scholar]
- Bienenstock J., Bloch K. J. Immunoconglutinin in various rheumatic diseases and certain diseases suspected of an autoimmune pathogenesis. Arthritis Rheum. 1967 Jun;10(3):187–198. doi: 10.1002/art.1780100304. [DOI] [PubMed] [Google Scholar]
- Bindon C. I., Hale G., Brüggemann M., Waldmann H. Human monoclonal IgG isotypes differ in complement activating function at the level of C4 as well as C1q. J Exp Med. 1988 Jul 1;168(1):127–142. doi: 10.1084/jem.168.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourke B. E., Moss I. K., Maini R. N. Measurement of the complement C3 breakdown product C3d by rocket immunoelectrophoresis. J Immunol Methods. 1982;48(1):97–108. doi: 10.1016/0022-1759(82)90214-9. [DOI] [PubMed] [Google Scholar]
- Brouwer E., Tervaert J. W., Horst G., Huitema M. G., van der Giessen M., Limburg P. C., Kallenberg C. G. Predominance of IgG1 and IgG4 subclasses of anti-neutrophil cytoplasmic autoantibodies (ANCA) in patients with Wegener's granulomatosis and clinically related disorders. Clin Exp Immunol. 1991 Mar;83(3):379–386. doi: 10.1111/j.1365-2249.1991.tb05647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druguet M., Chayvialle J. A., André C., Pepys M. B. Radioimmunoassay for immunoconglutinins. J Immunol Methods. 1980;34(3):181–190. doi: 10.1016/0022-1759(80)90045-9. [DOI] [PubMed] [Google Scholar]
- Durand C. G., Burge J. J. A new enzyme-linked immunosorbent assay (ELISA) for measuring immunoconglutinins directed against the third component of human complement. Findings in systemic lupus erythematosus. J Immunol Methods. 1984 Oct 12;73(1):57–66. doi: 10.1016/0022-1759(84)90031-0. [DOI] [PubMed] [Google Scholar]
- Ekdahl K. N., Nilsson U. R., Nilsson B. Inhibition of factor I by diisopropylfluorophosphate. Evidence of conformational changes in factor I induced by C3b and additional studies on the specificity of factor I. J Immunol. 1990 Jun 1;144(11):4269–4274. [PubMed] [Google Scholar]
- Erdei A., Füst G., Gergely J. The role of C3 in the immune response. Immunol Today. 1991 Sep;12(9):332–337. doi: 10.1016/0167-5699(91)90011-H. [DOI] [PubMed] [Google Scholar]
- Hammer C. H., Wirtz G. H., Renfer L., Gresham H. D., Tack B. F. Large scale isolation of functionally active components of the human complement system. J Biol Chem. 1981 Apr 25;256(8):3995–4006. [PubMed] [Google Scholar]
- Lachmann P. J. Conglutinin and immunoconglutinins. Adv Immunol. 1967;6:479–527. doi: 10.1016/s0065-2776(08)60527-1. [DOI] [PubMed] [Google Scholar]
- Laurell C. B. Quantitative estimation of proteins by electrophoresis in agarose gel containing antibodies. Anal Biochem. 1966 Apr;15(1):45–52. doi: 10.1016/0003-2697(66)90246-6. [DOI] [PubMed] [Google Scholar]
- Nilsson B., Ekdahl K. N., Svarvare M., Bjelle A., Nilsson U. R. Purification and characterization of IgG immunoconglutinins from patients with systemic lupus erythematosus: implications for a regulatory function. Clin Exp Immunol. 1990 Nov;82(2):262–267. doi: 10.1111/j.1365-2249.1990.tb05437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nossent J. C., Huysen V., Smeenk R. J., Swaak A. J. Low avidity antibodies to double stranded DNA in systemic lupus erythematosus: a longitudinal study of their clinical significance. Ann Rheum Dis. 1989 Aug;48(8):677–682. doi: 10.1136/ard.48.8.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orstavik K. H. Inhibitors to factor IX contain all IgG subclasses except IgG3. Folia Haematol Int Mag Klin Morphol Blutforsch. 1990;117(4):545–548. [PubMed] [Google Scholar]
- Shirakata Y., Shiraishi S., Sayama K., Miki Y. Subclass characteristics of IgG autoantibodies in bullous pemphigoid and pemphigus. J Dermatol. 1990 Nov;17(11):661–666. doi: 10.1111/j.1346-8138.1990.tb03008.x. [DOI] [PubMed] [Google Scholar]
- Sturfelt G., Sjöholm A. G. Complement components, complement activation, and acute phase response in systemic lupus erythematosus. Int Arch Allergy Appl Immunol. 1984;75(1):75–83. doi: 10.1159/000233593. [DOI] [PubMed] [Google Scholar]


