Abstract
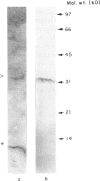
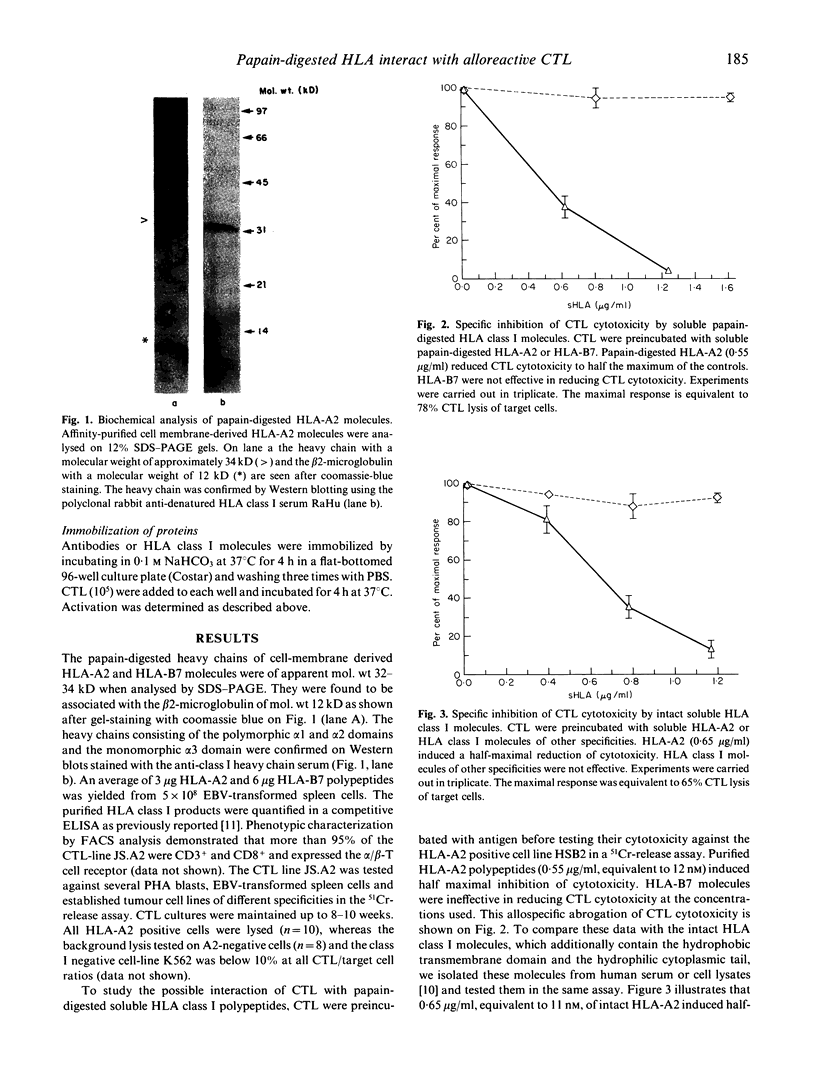
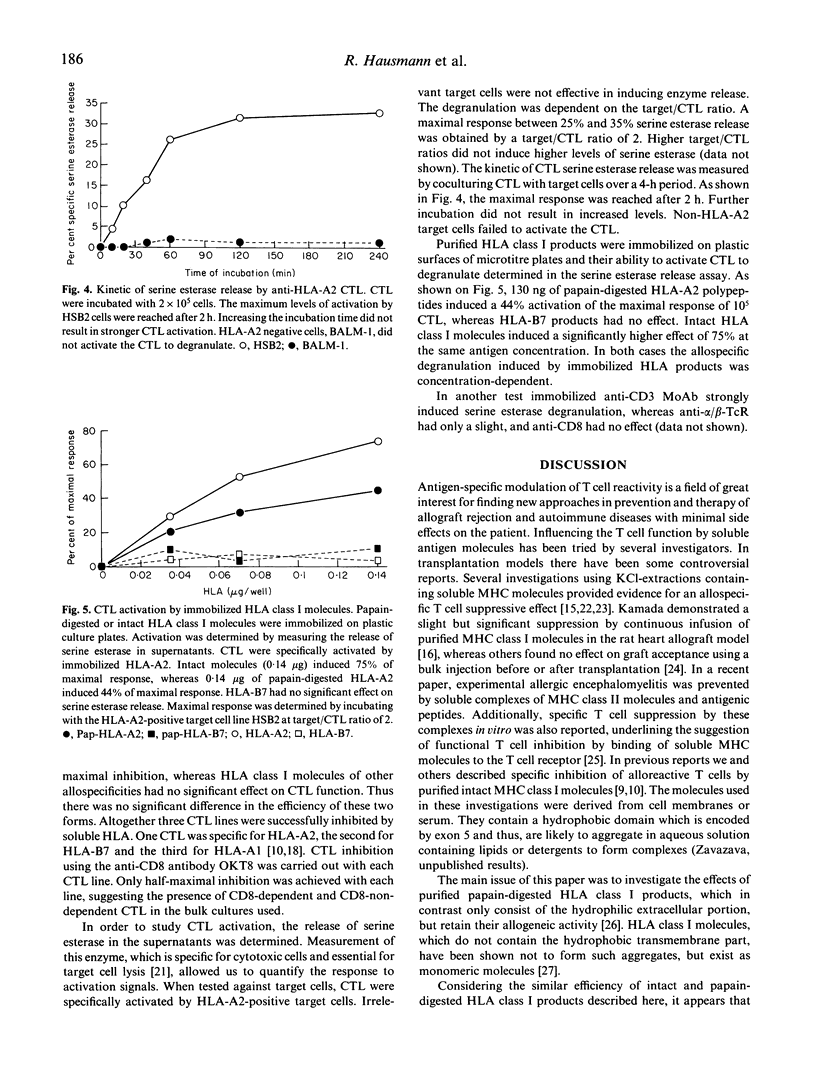
Acute immunological rejection events of transplanted allogeneic organs are strongly dependent on T cell reactivity against foreign MHC products. The recognition requirements of alloreactive cytotoxic T cells are of particular interest for finding approaches to modulating alloreactivity. The role of the allogeneic MHC molecule itself and/or an associated peptide in the interaction with the T cell receptor is still, however, unclear. Our studies have focused on the interactions of papain-digested HLA class I molecules with alloreactive CD8+ CTL. These polypeptides, consisting of the polymorphic alpha 1 and alpha 2 and the monomorphic alpha 3 domains, were used in both soluble and immobilized form to study their functional effects on anti-HLA-A2 reactive CTL. Purified polypeptides were of molecular mass 32-34 kD. HLA-A2 polypeptides (0.55 micrograms/ml) in soluble form induced half-maximal reduction of CTL cytotoxicity. These concentrations were quantitatively comparable to the effective doses of intact HLA class I molecules, which contain the hydrophobic transmembrane domain and the intracytoplasmic tail. In addition, specific activation requirements of these CTL were investigated in a serine esterase release assay. Maximal degranulation was observed after 2 h of antigen contact. Purified HLA class I molecules allospecifically activated the anti-HLA-A2 CTL to degranulate serine esterase, when immobilized on plastic microtitre plates. Thus, polypeptides containing the polymorphic alpha 1 and alpha 2 domains of human class I molecules potentially modulate the cytotoxic T cell response. This might have implications for the reduction or prevention of allograft rejection in recipients of foreign organs.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. Structure of the human class I histocompatibility antigen, HLA-A2. Nature. 1987 Oct 8;329(6139):506–512. doi: 10.1038/329506a0. [DOI] [PubMed] [Google Scholar]
- Connolly J. M., Hansen T. H., Ingold A. L., Potter T. A. Recognition by CD8 on cytotoxic T lymphocytes is ablated by several substitutions in the class I alpha 3 domain: CD8 and the T-cell receptor recognize the same class I molecule. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2137–2141. doi: 10.1073/pnas.87.6.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didlake R., Kim E. K., Kahan B. D. Ability of 3M KC1-extracted histocompatibility antigen to potentiate the immunosuppressive effect of cyclosporine to prolong the survival of heterotopic rat cardiac allografts. Transplantation. 1988 Nov;46(5):743–747. doi: 10.1097/00007890-198811000-00022. [DOI] [PubMed] [Google Scholar]
- Dobbe L. M., Stam N. J., Neefjes J. J., Giphart M. J. Biochemical complexity of serum HLA class I molecules. Immunogenetics. 1988;27(3):203–210. doi: 10.1007/BF00346587. [DOI] [PubMed] [Google Scholar]
- Elliott T. J., Eisen H. N. Allorecognition of purified major histocompatibility complex glycoproteins by cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2728–2732. doi: 10.1073/pnas.85.8.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk K., Rötzschke O., Rammensee H. G. Cellular peptide composition governed by major histocompatibility complex class I molecules. Nature. 1990 Nov 15;348(6298):248–251. doi: 10.1038/348248a0. [DOI] [PubMed] [Google Scholar]
- Hausmann R., Zavazava N., Müller-Ruchholtz W. Interaction of purified HLA class I molecules with alloreactive CTL. Transplant Proc. 1991 Aug;23(4):2255–2257. [PubMed] [Google Scholar]
- Heath W. R., Sherman L. A. Cell-type-specific recognition of allogeneic cells by alloreactive cytotoxic T cells: a consequence of peptide-dependent allorecognition. Eur J Immunol. 1991 Jan;21(1):153–159. doi: 10.1002/eji.1830210123. [DOI] [PubMed] [Google Scholar]
- Ito T., Stepkowski S. M., Kahan B. D. Soluble antigen and cyclosporine-induced specific unresponsiveness in rats. Frequency of alloantigen-specific T cytotoxic cells in normal, sensitized, and unresponsive rats. Transplantation. 1990 Feb;49(2):422–428. doi: 10.1097/00007890-199002000-00038. [DOI] [PubMed] [Google Scholar]
- Kane K. P., Mescher M. F. Antigen recognition by T cells. Quantitative effects of augmentation by antibodies providing accessory interactions. J Immunol. 1990 Feb 1;144(3):824–829. [PubMed] [Google Scholar]
- Kane K. P., Sherman L. A., Mescher M. F. Molecular interactions required for triggering alloantigen-specific cytolytic T lymphocytes. J Immunol. 1989 Jun 15;142(12):4153–4160. [PubMed] [Google Scholar]
- Krangel M. S., Pious D., Strominger J. L. Characterization of a B lymphoblastoid cell line mutant that secretes HLA-A2. J Immunol. 1984 Jun;132(6):2984–2991. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marrack P., Kappler J. T cells can distinguish between allogeneic major histocompatibility complex products on different cell types. Nature. 1988 Apr 28;332(6167):840–843. doi: 10.1038/332840a0. [DOI] [PubMed] [Google Scholar]
- Müllbacher A., Hill A. B., Blanden R. V., Cowden W. B., King N. J., Hla R. T. Alloreactive cytotoxic T cells recognize MHC class I antigen without peptide specificity. J Immunol. 1991 Sep 15;147(6):1765–1772. [PubMed] [Google Scholar]
- Parham P., Alpert B. N., Orr H. T., Strominger J. L. Carbohydrate moiety of HLA antigens. Antigenic properties and amino acid sequences around the site of glycosylation. J Biol Chem. 1977 Nov 10;252(21):7555–7567. [PubMed] [Google Scholar]
- Parham P., Clayberger C., Zorn S. L., Ludwig D. S., Schoolnik G. K., Krensky A. M. Inhibition of alloreactive cytotoxic T lymphocytes by peptides from the alpha 2 domain of HLA-A2. Nature. 1987 Feb 12;325(6105):625–628. doi: 10.1038/325625a0. [DOI] [PubMed] [Google Scholar]
- Pasternack M. S., Eisen H. N. A novel serine esterase expressed by cytotoxic T lymphocytes. 1985 Apr 25-May 1Nature. 314(6013):743–745. doi: 10.1038/314743a0. [DOI] [PubMed] [Google Scholar]
- Priestley C. A., Dalchau R., Sawyer G. J., Fabre J. W. A detailed analysis of the potential of water soluble classical class I MHC molecules for donor-specific immunosuppression in transplantation. Transplant Proc. 1990 Aug;22(4):1949–1951. [PubMed] [Google Scholar]
- Priestley C. A., Dalchau R., Sawyer G. J., Fabre J. W. A detailed analysis of the potential of water-soluble classical class I MHC molecules for the suppression of kidney allograft rejection and in vitro cytotoxic T cell responses. Transplantation. 1989 Dec;48(6):1031–1038. doi: 10.1097/00007890-198912000-00028. [DOI] [PubMed] [Google Scholar]
- Salter R. D., Benjamin R. J., Wesley P. K., Buxton S. E., Garrett T. P., Clayberger C., Krensky A. M., Norment A. M., Littman D. R., Parham P. A binding site for the T-cell co-receptor CD8 on the alpha 3 domain of HLA-A2. Nature. 1990 May 3;345(6270):41–46. doi: 10.1038/345041a0. [DOI] [PubMed] [Google Scholar]
- Schneck J., Maloy W. L., Coligan J. E., Margulies D. H. Inhibition of an allospecific T cell hybridoma by soluble class I proteins and peptides: estimation of the affinity of a T cell receptor for MHC. Cell. 1989 Jan 13;56(1):47–55. doi: 10.1016/0092-8674(89)90982-3. [DOI] [PubMed] [Google Scholar]
- Schneck J., Munitz T., Coligan J. E., Maloy W. L., Margulies D. H., Singer A. Inhibition of allorecognition by an H-2Kb-derived peptide is evidence for a T-cell binding region on a major histocompatibility complex molecule. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8516–8520. doi: 10.1073/pnas.86.21.8516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S. D., Nag B., Su X. M., Green D., Spack E., Clark B. R., Sriram S. Antigen-specific therapy of experimental allergic encephalomyelitis by soluble class II major histocompatibility complex-peptide complexes. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11465–11469. doi: 10.1073/pnas.88.24.11465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumimoto R., Kamada N. Specific suppression of allograft rejection by soluble class I antigen and complexes with monoclonal antibody. Transplantation. 1990 Oct;50(4):678–682. doi: 10.1097/00007890-199010000-00029. [DOI] [PubMed] [Google Scholar]
- Turner M. J., Cresswell P., Parham P., Strominger J. L., Mann D. L., Sanderson A. R. Purification of papain-solubilized histocompatibility antigens from a cultured human lymphoblastoid line, RPMI 4265. J Biol Chem. 1975 Jun 25;250(12):4512–4519. [PubMed] [Google Scholar]
- Yasumura T., Kahan B. D. Prolongation of rat kidney allografts by pretransplant administration of donor antigen extract or whole blood transfusion combined with a short course of cyclosporine. Transplantation. 1983 Dec;36(6):603–609. doi: 10.1097/00007890-198336060-00002. [DOI] [PubMed] [Google Scholar]
- Zavazava N., Hausmann R., Müller-Ruchholtz W. Inhibition of anti-HLA-B7 alloreactive CTL by affinity-purified soluble HLA. Transplantation. 1991 Apr;51(4):838–842. doi: 10.1097/00007890-199104000-00019. [DOI] [PubMed] [Google Scholar]
- Zavazava N., Westphal E., Müller-Ruchholtz W. Characterization of soluble HLA molecules in sweat and quantitative HLA differences in serum of healthy individuals. J Immunogenet. 1990 Dec;17(6):387–394. doi: 10.1111/j.1744-313x.1990.tb00890.x. [DOI] [PubMed] [Google Scholar]
- van Rood J. J., van Leeuwen A., van Santen M. C. Anti HL-A2 inhibitor in normal human serum. Nature. 1970 Apr 25;226(5243):366–367. doi: 10.1038/226366a0. [DOI] [PubMed] [Google Scholar]