Abstract
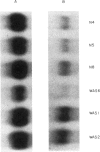
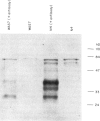
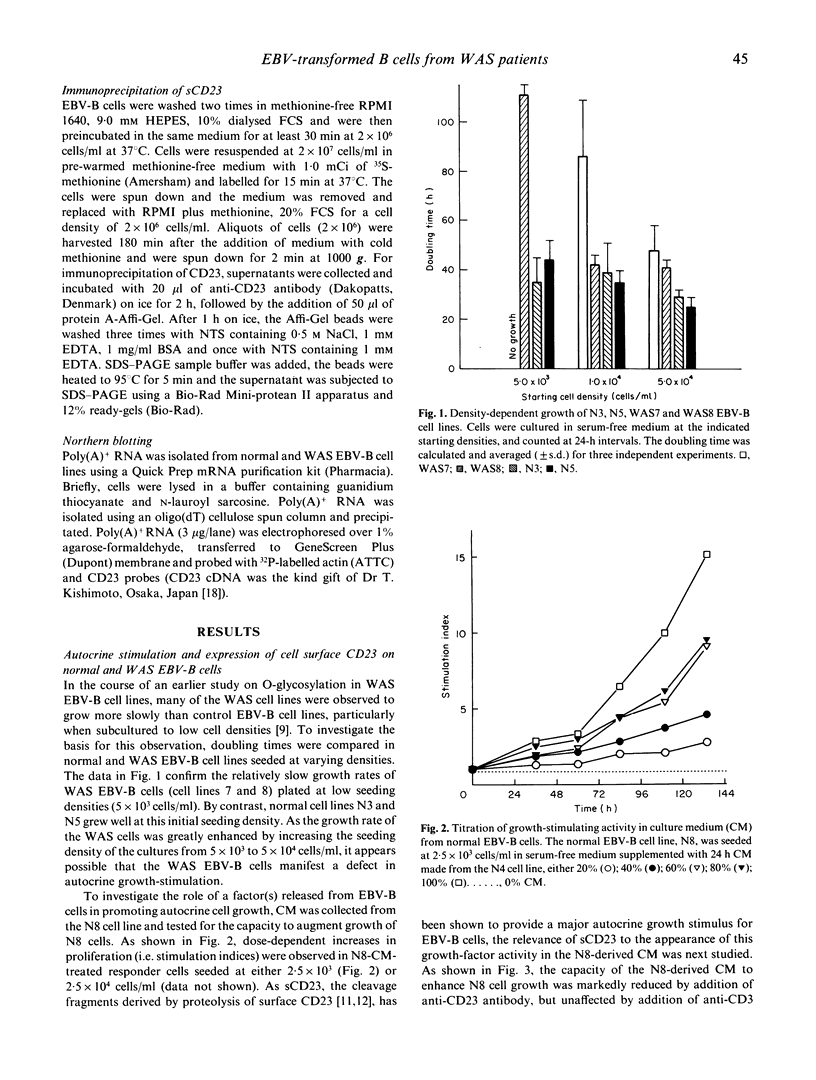
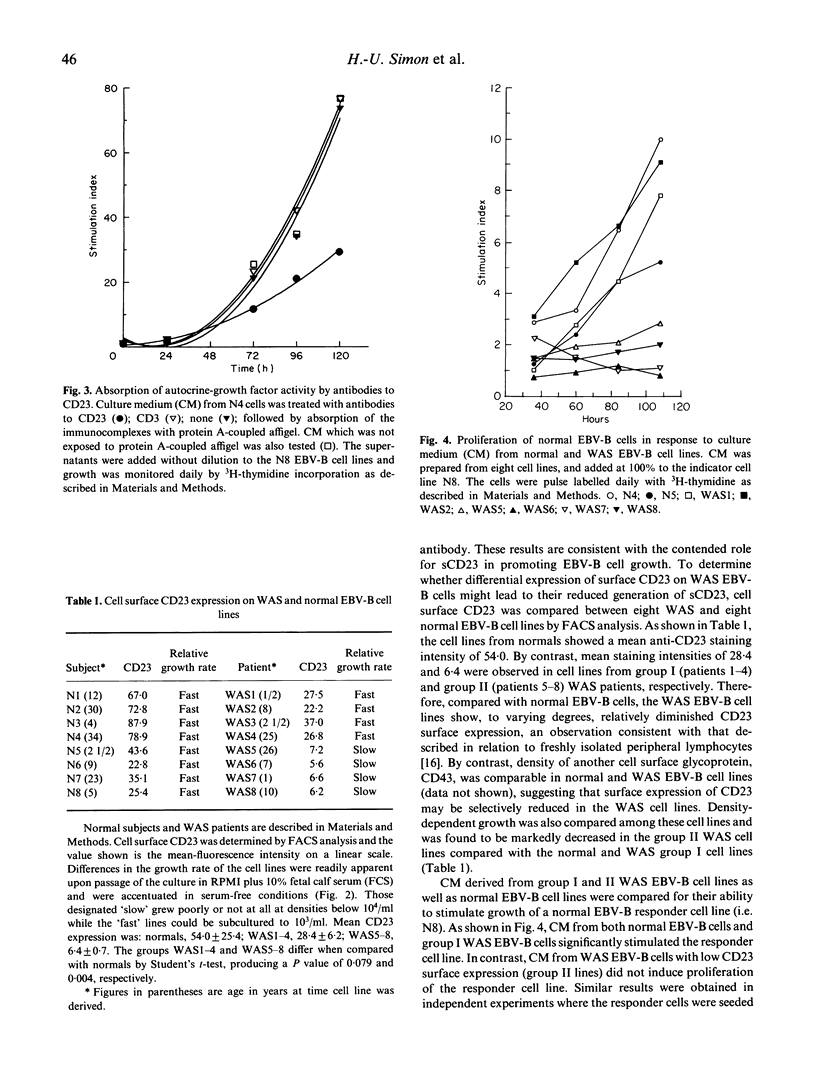
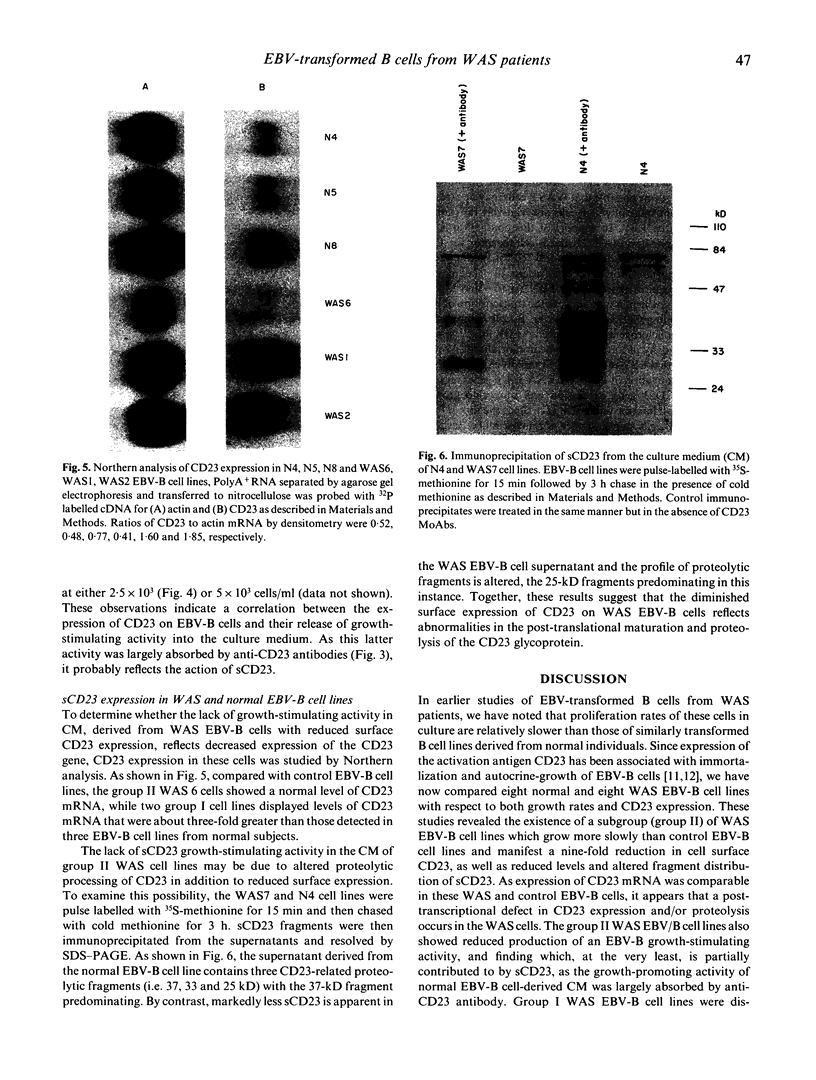
WAS is an X-linked, recessive, immune deficiency syndrome, characteristically associated with lymphocyte and platelet dysfunction. Peripheral B lymphocytes from WAS patients are nonresponsive to polysaccharide antigens and show reduced numbers of cells expressing the integral membrane glycoprotein, CD23. The release of CD23 proteolytic fragments, so-called soluble CD23 (sCD23), by B lymphoblasts and EBV-transformed B cell lines has previously been described, and these fragments have been shown to stimulate autocrine growth of these cells. We have found that the surface expression of CD23 is reduced on WAS compared with control EBV-B cells. Surface CD23 levels were reduced two-fold in four WAS cell lines (group I) and nine-fold in four other lines (group II). Group II WAS cell lines also showed reduced growth rates in serum-free medium when compared with group I cell lines and EBV-B cell lines from eight normal subjects. In contrast to the group II WAS lines, group I and EBV-B cells from normal individuals produced an autocrine-growth factor activity which could be absorbed by anti-CD23 antibodies. Immunoprecipitation of sCD23 from culture supernatants confirmed that group I WAS cell lines produced less sCD23, particularly the 37K fragment which was prevalent in control EBV-B cells. Northern analysis showed that CD23 mRNA levels were increased three-fold in group I and unchanged in group II WAS compared with normal EBV-B cell lines, suggesting that decreased surface expression in WAS EBV-B cells reflects post-transcriptional events. Together these results suggest that reduced cell surface expression and aberrant proteolysis of CD23 occurs in WAS patients' B lymphocytes and may contribute to impaired immune function in these patients.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALDRICH R. A., STEINBERG A. G., CAMPBELL D. C. Pedigree demonstrating a sex-linked recessive condition characterized by draining ears, eczematoid dermatitis and bloody diarrhea. Pediatrics. 1954 Feb;13(2):133–139. [PubMed] [Google Scholar]
- Azim T., Allday M. J., Crawford D. H. Immortalization of Epstein-Barr virus-infected CD23-negative B lymphocytes by the addition of B cell growth factor. J Gen Virol. 1990 Mar;71(Pt 3):665–671. doi: 10.1099/0022-1317-71-3-665. [DOI] [PubMed] [Google Scholar]
- Azim T., Crawford D. H. Lymphocytes activated by the Epstein-Barr virus to produce immunoglobulin do not express CD23 or become immortalized. Int J Cancer. 1988 Jul 15;42(1):23–28. doi: 10.1002/ijc.2910420106. [DOI] [PubMed] [Google Scholar]
- Blaese R. M., Strober W., Brown R. S., Waldmann T. A. The Wiskott-Aldrich syndrome. A disorder with a possible defect in antigen processing or recognition. Lancet. 1968 May 18;1(7551):1056–1061. doi: 10.1016/s0140-6736(68)91411-6. [DOI] [PubMed] [Google Scholar]
- Garcia-Blanco M. A., Cullen B. R. Molecular basis of latency in pathogenic human viruses. Science. 1991 Nov 8;254(5033):815–820. doi: 10.1126/science.1658933. [DOI] [PubMed] [Google Scholar]
- Golding B., Muchmore A. V., Blaese R. M. Newborn and Wiskott-Aldrich patient B cells can be activated by TNP-Brucella abortus: evidence that TNP-Brucella abortus behaves as a T-independent type 1 antigen in humans. J Immunol. 1984 Dec;133(6):2966–2971. [PubMed] [Google Scholar]
- Gordon J., Aman P., Rosén A., Ernberg I., Ehlin-Henriksson B., Klein G. Capacity of B-lymphocytic lines of diverse tumor origin to produce and respond to B-cell growth factors: a progression model for B-cell lymphomagenesis. Int J Cancer. 1985 Feb 15;35(2):251–256. doi: 10.1002/ijc.2910350218. [DOI] [PubMed] [Google Scholar]
- Gordon J., Flores-Romo L., Cairns J. A., Millsum M. J., Lane P. J., Johnson G. D., MacLennan I. C. CD23: a multi-functional receptor/lymphokine? Immunol Today. 1989 May;10(5):153–157. doi: 10.1016/0167-5699(89)90171-0. [DOI] [PubMed] [Google Scholar]
- Gordon J., Liu Y. J., MacLennan I. C., Flores-Romo L., Shields J., Bonnefoy J. Y. CD23 and immune modulation. Immunol Today. 1991 Jun;12(6):206–206. doi: 10.1016/0167-5699(91)90055-X. [DOI] [PubMed] [Google Scholar]
- HUNTLEY C. C., DEES S. C. Eczema associated with thrombocytopenic purpura and purulent otitis media; report of five fatal cases. Pediatrics. 1957 Mar;19(3):351–361. [PubMed] [Google Scholar]
- Higgins E. A., Siminovitch K. A., Zhuang D. L., Brockhausen I., Dennis J. W. Aberrant O-linked oligosaccharide biosynthesis in lymphocytes and platelets from patients with the Wiskott-Aldrich syndrome. J Biol Chem. 1991 Apr 5;266(10):6280–6290. [PubMed] [Google Scholar]
- Keegan A. D., Conrad D. H. The murine lymphocyte receptor for IgE. V. Biosynthesis, transport, and maturation of the B cell Fc epsilon receptor. J Immunol. 1987 Aug 15;139(4):1199–1205. [PubMed] [Google Scholar]
- Kikutani H., Inui S., Sato R., Barsumian E. L., Owaki H., Yamasaki K., Kaisho T., Uchibayashi N., Hardy R. R., Hirano T. Molecular structure of human lymphocyte receptor for immunoglobulin E. Cell. 1986 Dec 5;47(5):657–665. doi: 10.1016/0092-8674(86)90508-8. [DOI] [PubMed] [Google Scholar]
- Kingsley D. M., Kozarsky K. F., Hobbie L., Krieger M. Reversible defects in O-linked glycosylation and LDL receptor expression in a UDP-Gal/UDP-GalNAc 4-epimerase deficient mutant. Cell. 1986 Mar 14;44(5):749–759. doi: 10.1016/0092-8674(86)90841-x. [DOI] [PubMed] [Google Scholar]
- Kozarsky K. F., Call S. M., Dower S. K., Krieger M. Abnormal intracellular sorting of O-linked carbohydrate-deficient interleukin-2 receptors. Mol Cell Biol. 1988 Aug;8(8):3357–3363. doi: 10.1128/mcb.8.8.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W. T., Rao M., Conrad D. H. The murine lymphocyte receptor for IgE. IV. The mechanism of ligand-specific receptor upregulation on B cells. J Immunol. 1987 Aug 15;139(4):1191–1198. [PubMed] [Google Scholar]
- Nemerow G. R., Moore M. D., Cooper N. R. Structure and function of the B-lymphocyte Epstein-Barr virus/C3d receptor. Adv Cancer Res. 1990;54:273–300. doi: 10.1016/s0065-230x(08)60814-3. [DOI] [PubMed] [Google Scholar]
- Ochs H. D., Slichter S. J., Harker L. A., Von Behrens W. E., Clark R. A., Wedgwood R. J. The Wiskott-Aldrich syndrome: studies of lymphocytes, granulocytes, and platelets. Blood. 1980 Feb;55(2):243–252. [PubMed] [Google Scholar]
- Oppenheim J. J., Blaese R. M., Waldmann T. A. Defective lymphocyte transformation and delayed hypersensitivity in Wiskott-Aldrich syndrome. J Immunol. 1970 Apr;104(4):835–844. [PubMed] [Google Scholar]
- Piller F., Le Deist F., Weinberg K. I., Parkman R., Fukuda M. Altered O-glycan synthesis in lymphocytes from patients with Wiskott-Aldrich syndrome. J Exp Med. 1991 Jun 1;173(6):1501–1510. doi: 10.1084/jem.173.6.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piller F., Piller V., Fox R. I., Fukuda M. Human T-lymphocyte activation is associated with changes in O-glycan biosynthesis. J Biol Chem. 1988 Oct 15;263(29):15146–15150. [PubMed] [Google Scholar]
- Spitler L. E., Levin A. S., Stites D. P., Fudenberg H. H., Huber H. The Wiskott-Aldrich syndrome. Immunologic studies in nine patients and selected family members. Cell Immunol. 1975 Oct;19(2):201–218. doi: 10.1016/0008-8749(75)90204-x. [DOI] [PubMed] [Google Scholar]
- Swendeman S., Thorley-Lawson D. A. The activation antigen BLAST-2, when shed, is an autocrine BCGF for normal and transformed B cells. EMBO J. 1987 Jun;6(6):1637–1642. doi: 10.1002/j.1460-2075.1987.tb02412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchibayashi N., Kikutani H., Barsumian E. L., Hauptmann R., Schneider F. J., Schwendenwein R., Sommergruber W., Spevak W., Maurer-Fogy I., Suemura M. Recombinant soluble Fc epsilon receptor II (Fc epsilon RII/CD23) has IgE binding activity but no B cell growth promoting activity. J Immunol. 1989 Jun 1;142(11):3901–3908. [PubMed] [Google Scholar]
- Yokota A., Kikutani H., Tanaka T., Sato R., Barsumian E. L., Suemura M., Kishimoto T. Two species of human Fc epsilon receptor II (Fc epsilon RII/CD23): tissue-specific and IL-4-specific regulation of gene expression. Cell. 1988 Nov 18;55(4):611–618. doi: 10.1016/0092-8674(88)90219-x. [DOI] [PubMed] [Google Scholar]