Abstract
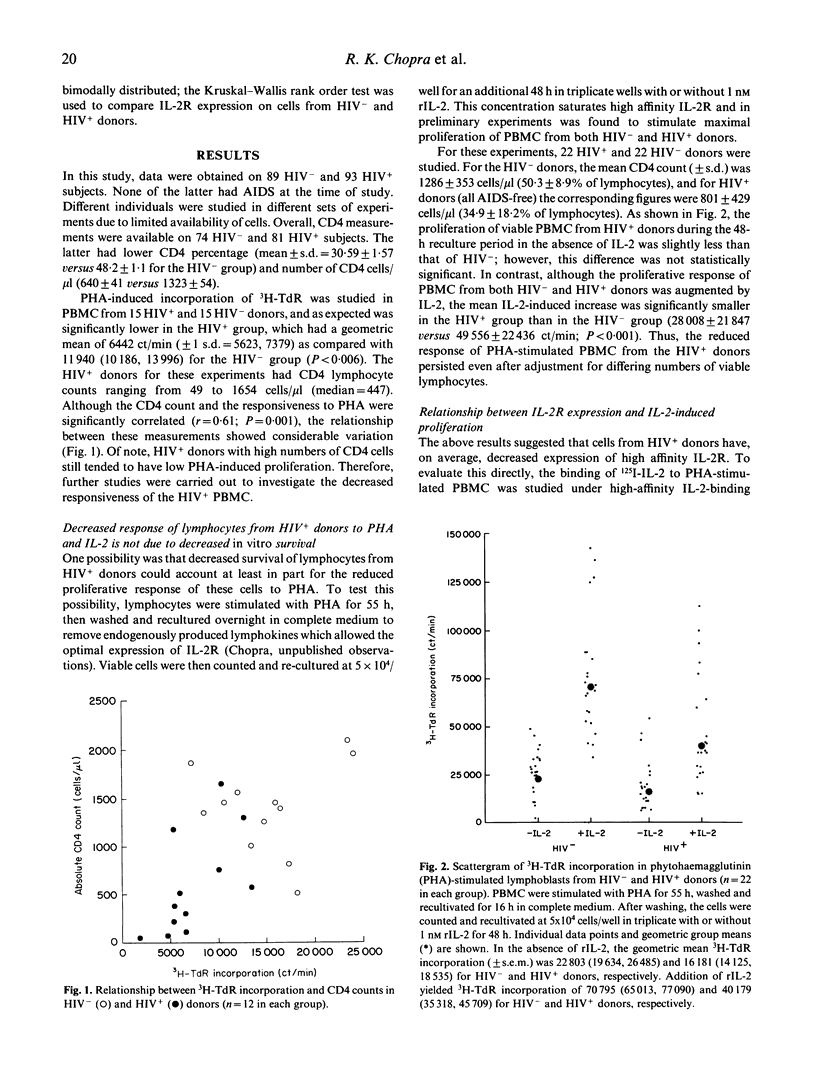
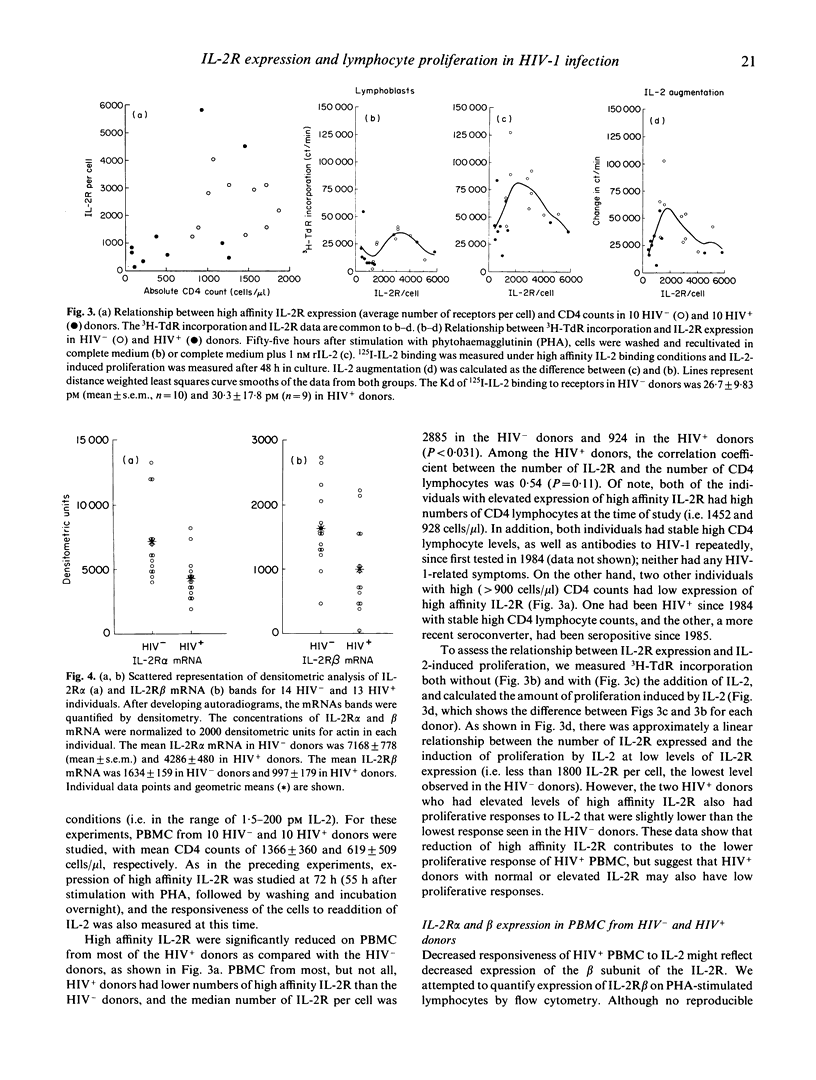
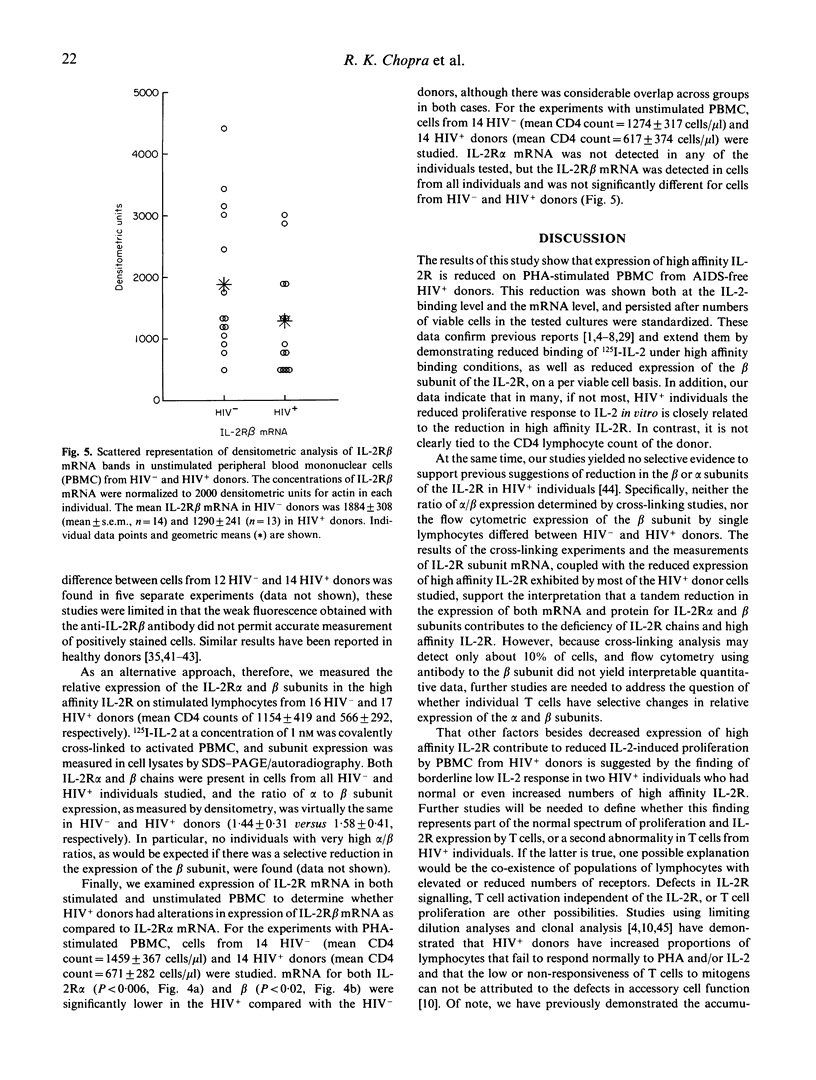
Previous studies have shown that exogenous IL-2 does not correct the reduction in phytohaemagglutinin (PHA)-induced proliferation of lymphocytes from HIV-1 infected (HIV+) individuals. We investigated the mechanism of this reduction to determine if reduced expression of the complete IL-2 receptor (IL-2R) was responsible. In a series of experiments, PHA-stimulated lymphocytes from a total of 89 HIV- and 93 HIV+ homosexual men from the Baltimore Multicentre AIDS Cohort Study (MACS) were studied to determine the expression of messages for the alpha and beta subunits of the IL-2R, the binding of 125I-IL-2 to high affinity IL-2R, and the effect of IL-2 on cell proliferation. Compared to HIV- donors, PHA-stimulated lymphocytes from most HIV+ donors demonstrated (i) a reduction in high affinity IL-2R expression that correlated with the reduction in the IL-2-induced proliferative response; and (ii) a reduction in expression of both IL-2R alpha- and beta-chain mRNA which may be responsible for decreased high affinity IL-2R expression. However, lymphocytes from some HIV+ individuals had borderline low IL-2-induced proliferation despite normal or elevated expression of high affinity IL-2R. These results suggest that decreased expression of IL-2R may account, at least in part, for the lower proliferative response of cells from HIV+ donors.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bich-Thuy L. T., Dukovich M., Peffer N. J., Fauci A. S., Kehrl J. H., Greene W. C. Direct activation of human resting T cells by IL 2: the role of an IL 2 receptor distinct from the Tac protein. J Immunol. 1987 Sep 1;139(5):1550–1556. [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Chopra R. K., Powers D. C., Kendig N. E., Adler W. H., Nagel J. E. Soluble interleukin 2 receptors released from mitogen stimulated human peripheral blood lymphocytes bind interleukin 2 and inhibit IL2 dependent cell proliferation. Immunol Invest. 1989 Oct;18(8):961–973. doi: 10.3109/08820138909045783. [DOI] [PubMed] [Google Scholar]
- Cianciolo G. J., Copeland T. D., Oroszlan S., Snyderman R. Inhibition of lymphocyte proliferation by a synthetic peptide homologous to retroviral envelope proteins. Science. 1985 Oct 25;230(4724):453–455. doi: 10.1126/science.2996136. [DOI] [PubMed] [Google Scholar]
- Ciobanu N., Welte K., Kruger G., Venuta S., Gold J., Feldman S. P., Wang C. Y., Koziner B., Moore M. A., Safai B. Defective T-cell response to PHA and mitogenic monoclonal antibodies in male homosexuals with acquired immunodeficiency syndrome and its in vitro correction by interleukin 2. J Clin Immunol. 1983 Oct;3(4):332–340. doi: 10.1007/BF00915794. [DOI] [PubMed] [Google Scholar]
- Donnenberg A. D., Margolick J. B., Polk B. F. Limiting dilution analysis of in vivo-activated (IL-2 responsive) peripheral blood lymphocytes in HIV-1-infected subjects. Clin Immunol Immunopathol. 1989 Apr;51(1):91–98. doi: 10.1016/0090-1229(89)90209-2. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Giorgi J. V., Cheng H. L., Margolick J. B., Bauer K. D., Ferbas J., Waxdal M., Schmid I., Hultin L. E., Jackson A. L., Park L. Quality control in the flow cytometric measurement of T-lymphocyte subsets: the multicenter AIDS cohort study experience. The Multicenter AIDS Cohort Study Group. Clin Immunol Immunopathol. 1990 May;55(2):173–186. doi: 10.1016/0090-1229(90)90096-9. [DOI] [PubMed] [Google Scholar]
- Giorgi J. V., Fahey J. L., Smith D. C., Hultin L. E., Cheng H. L., Mitsuyasu R. T., Detels R. Early effects of HIV on CD4 lymphocytes in vivo. J Immunol. 1987 Jun 1;138(11):3725–3730. [PubMed] [Google Scholar]
- Greene W. C. The human interleukin-2 receptor: a molecular and biochemical analysis of structure and function. Clin Res. 1987 Sep;35(5):439–450. [PubMed] [Google Scholar]
- Harper M. E., Marselle L. M., Gallo R. C., Wong-Staal F. Detection of lymphocytes expressing human T-lymphotropic virus type III in lymph nodes and peripheral blood from infected individuals by in situ hybridization. Proc Natl Acad Sci U S A. 1986 Feb;83(3):772–776. doi: 10.1073/pnas.83.3.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatakeyama M., Tsudo M., Minamoto S., Kono T., Doi T., Miyata T., Miyasaka M., Taniguchi T. Interleukin-2 receptor beta chain gene: generation of three receptor forms by cloned human alpha and beta chain cDNA's. Science. 1989 May 5;244(4904):551–556. doi: 10.1126/science.2785715. [DOI] [PubMed] [Google Scholar]
- Hauser G. J., Bino T., Rosenberg H., Zakuth V., Geller E., Spirer Z. Interleukin-2 production and response to exogenous interleukin-2 in a patient with the acquired immune deficiency syndrome (AIDS). Clin Exp Immunol. 1984 Apr;56(1):14–17. [PMC free article] [PubMed] [Google Scholar]
- Hoffman R. A., Kung P. C., Hansen W. P., Goldstein G. Simple and rapid measurement of human T lymphocytes and their subclasses in peripheral blood. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4914–4917. doi: 10.1073/pnas.77.8.4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann B., Moller J., Langhoff E., Jakobsen K. D., Odum N., Dickmeiss E., Ryder L. P., Thastrup O., Scharff O., Foder B. Stimulation of AIDS lymphocytes with calcium ionophore (A23187) and phorbol ester (PMA): studies of cytoplasmic free Ca, IL-2 receptor expression, IL-2 production, and proliferation. Cell Immunol. 1989 Mar;119(1):14–21. doi: 10.1016/0008-8749(89)90219-0. [DOI] [PubMed] [Google Scholar]
- Israel-Biet D., Ekwalanga M., Venet A., Even P., Andrieu J. M. Serum suppressive activity of HIV seropositive patients. Clin Exp Immunol. 1988 Nov;74(2):185–189. [PMC free article] [PubMed] [Google Scholar]
- Kaslow R. A., Ostrow D. G., Detels R., Phair J. P., Polk B. F., Rinaldo C. R., Jr The Multicenter AIDS Cohort Study: rationale, organization, and selected characteristics of the participants. Am J Epidemiol. 1987 Aug;126(2):310–318. doi: 10.1093/aje/126.2.310. [DOI] [PubMed] [Google Scholar]
- Kekow J., Wachsman W., McCutchan J. A., Cronin M., Carson D. A., Lotz M. Transforming growth factor beta and noncytopathic mechanisms of immunodeficiency in human immunodeficiency virus infection. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8321–8325. doi: 10.1073/pnas.87.21.8321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane H. C., Depper J. M., Greene W. C., Whalen G., Waldmann T. A., Fauci A. S. Qualitative analysis of immune function in patients with the acquired immunodeficiency syndrome. Evidence for a selective defect in soluble antigen recognition. N Engl J Med. 1985 Jul 11;313(2):79–84. doi: 10.1056/NEJM198507113130204. [DOI] [PubMed] [Google Scholar]
- Laurence J., Gottlieb A. B., Kunkel H. G. Soluble suppressor factors in patients with acquired immune deficiency syndrome and its prodrome. Elaboration in vitro by T lymphocyte-adherent cell interactions. J Clin Invest. 1983 Dec;72(6):2072–2081. doi: 10.1172/JCI111172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi E., Macchia D., Parronchi P., Mazzetti M., Ravina A., Milo D., Romagnani S. Reduced production of interleukin 2 and interferon-gamma and enhanced helper activity for IgG synthesis by cloned CD4+ T cells from patients with AIDS. Eur J Immunol. 1987 Dec;17(12):1685–1690. doi: 10.1002/eji.1830171202. [DOI] [PubMed] [Google Scholar]
- Margolick J. B., Volkman D. J., Lane H. C., Fauci A. S. Clonal analysis of T lymphocytes in the acquired immunodeficiency syndrome. Evidence for an abnormality affecting individual helper and suppressor T cells. J Clin Invest. 1985 Aug;76(2):709–715. doi: 10.1172/JCI112025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Rubin B. Y., Masur H., Roberts R. B. Impaired production of lymphokines and immune (gamma) interferon in the acquired immunodeficiency syndrome. N Engl J Med. 1984 Apr 5;310(14):883–889. doi: 10.1056/NEJM198404053101404. [DOI] [PubMed] [Google Scholar]
- Nair M. P., Pottathil R., Heimer E. P., Schwartz S. A. Immunoregulatory activities of human immunodeficiency virus (HIV) proteins: effect of HIV recombinant and synthetic peptides on immunoglobulin synthesis and proliferative responses by normal lymphocytes. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6498–6502. doi: 10.1073/pnas.85.17.6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi Y., Takeshita T., Nagata K., Mori S., Sugamura K. Differential expression of the IL-2 receptor subunits, p55 and p75 on various populations of primary peripheral blood mononuclear cells. J Immunol. 1989 Dec 1;143(11):3548–3555. [PubMed] [Google Scholar]
- Pantaleo G., Koenig S., Baseler M., Lane H. C., Fauci A. S. Defective clonogenic potential of CD8+ T lymphocytes in patients with AIDS. Expansion in vivo of a nonclonogenic CD3+CD8+DR+CD25- T cell population. J Immunol. 1990 Mar 1;144(5):1696–1704. [PubMed] [Google Scholar]
- Phillips J. H., Takeshita T., Sugamura K., Lanier L. L. Activation of natural killer cells via the p75 interleukin 2 receptor. J Exp Med. 1989 Jul 1;170(1):291–296. doi: 10.1084/jem.170.1.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince H. E., Czaplicki C. D. In vitro activation of T lymphocytes from HIV-seropositive blood donors. II. Decreased mitogen-induced expression of interleukin 2 receptor by both CD4 and CD8 cell subsets. Clin Immunol Immunopathol. 1988 Aug;48(2):132–139. doi: 10.1016/0090-1229(88)90077-3. [DOI] [PubMed] [Google Scholar]
- Prince H. E., Kermani-Arab V., Fahey J. L. Depressed interleukin 2 receptor expression in acquired immune deficiency and lymphadenopathy syndromes. J Immunol. 1984 Sep;133(3):1313–1317. [PubMed] [Google Scholar]
- Psallidopoulos M. C., Schnittman S. M., Thompson L. M., 3rd, Baseler M., Fauci A. S., Lane H. C., Salzman N. P. Integrated proviral human immunodeficiency virus type 1 is present in CD4+ peripheral blood lymphocytes in healthy seropositive individuals. J Virol. 1989 Nov;63(11):4626–4631. doi: 10.1128/jvi.63.11.4626-4631.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj N. B., Pitha P. M. Two levels of regulation of beta-interferon gene expression in human cells. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3923–3927. doi: 10.1073/pnas.80.13.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed J. C., Alpers J. D., Nowell P. C., Hoover R. G. Sequential expression of protooncogenes during lectin-stimulated mitogenesis of normal human lymphocytes. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3982–3986. doi: 10.1073/pnas.83.11.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb R. J., Mayer P. C., Garlick R. Retention of biological activity following radioiodination of human interleukin 2: comparison with biosynthetically labeled growth factor in receptor binding assays. J Immunol Methods. 1985 Jul 16;81(1):15–30. doi: 10.1016/0022-1759(85)90118-8. [DOI] [PubMed] [Google Scholar]
- Sethi K. K., Näher H. Elevated titers of cell-free interleukin-2 receptor in serum and cerebrospinal fluid specimens of patients with acquired immunodeficiency syndrome. Immunol Lett. 1986 Oct;13(4):179–184. doi: 10.1016/0165-2478(86)90052-0. [DOI] [PubMed] [Google Scholar]
- Smith K. A. Interleukin-2: inception, impact, and implications. Science. 1988 May 27;240(4856):1169–1176. doi: 10.1126/science.3131876. [DOI] [PubMed] [Google Scholar]
- Takeshita T., Goto Y., Tada K., Nagata K., Asao H., Sugamura K. Monoclonal antibody defining a molecule possibly identical to the p75 subunit of interleukin 2 receptor. J Exp Med. 1989 Apr 1;169(4):1323–1332. doi: 10.1084/jem.169.4.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang K. Y., Fudenberg H. H., Galbraith G. M., Donnelly R. P., Bishop L. R., Koopmann W. R. Partial restoration of impaired interleukin-2 production and Tac antigen (putative interleukin-2 receptor) expression in patients with acquired immune deficiency syndrome by isoprinosine treatment in vitro. J Clin Invest. 1985 May;75(5):1538–1544. doi: 10.1172/JCI111858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsudo M., Kitamura F., Miyasaka M. Characterization of the interleukin 2 receptor beta chain using three distinct monoclonal antibodies. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1982–1986. doi: 10.1073/pnas.86.6.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viscidi R. P., Mayur K., Lederman H. M., Frankel A. D. Inhibition of antigen-induced lymphocyte proliferation by Tat protein from HIV-1. Science. 1989 Dec 22;246(4937):1606–1608. doi: 10.1126/science.2556795. [DOI] [PubMed] [Google Scholar]
- Yagita H., Nakata M., Azuma A., Nitta T., Takeshita T., Sugamura K., Okumura K. Activation of peripheral blood T cells via the p75 interleukin 2 receptor. J Exp Med. 1989 Oct 1;170(4):1445–1450. doi: 10.1084/jem.170.4.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zola H., Koh L. Y., Mantzioris B. X., Rhodes D. Patients with HIV infection have a reduced proportion of lymphocytes expressing the IL2 receptor p55 chain (TAC, CD25). Clin Immunol Immunopathol. 1991 Apr;59(1):16–25. doi: 10.1016/0090-1229(91)90078-o. [DOI] [PubMed] [Google Scholar]


