Abstract
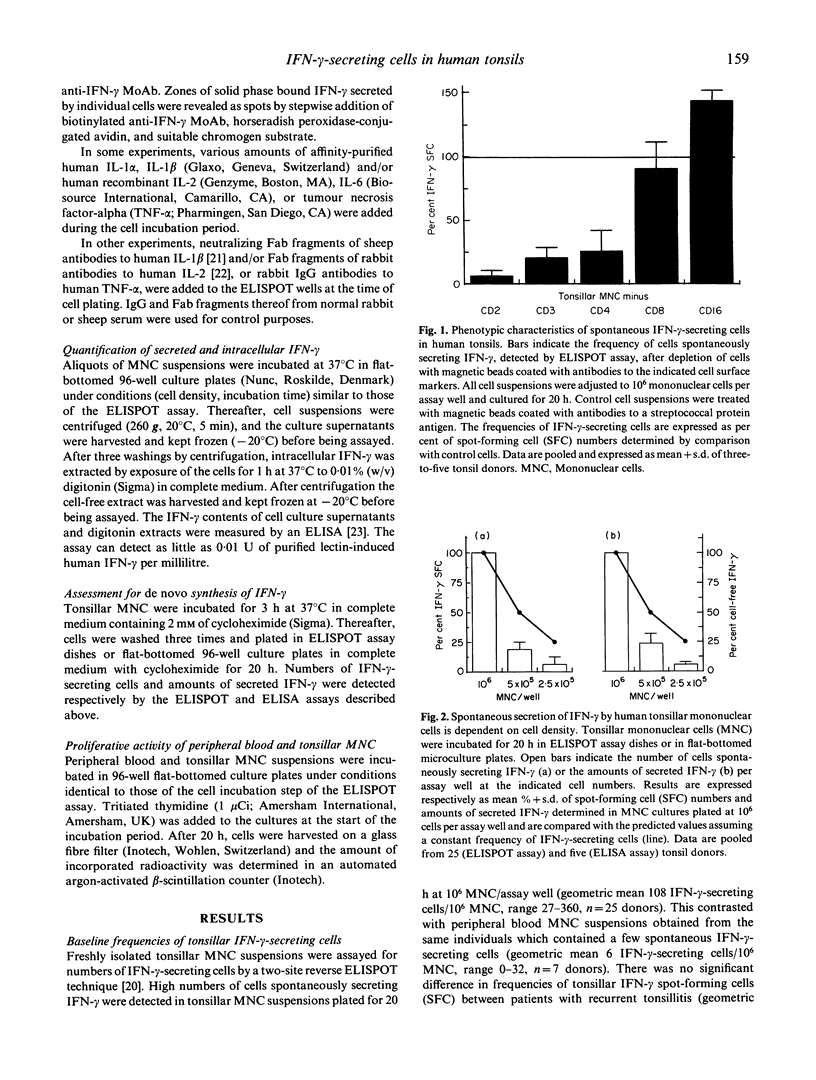
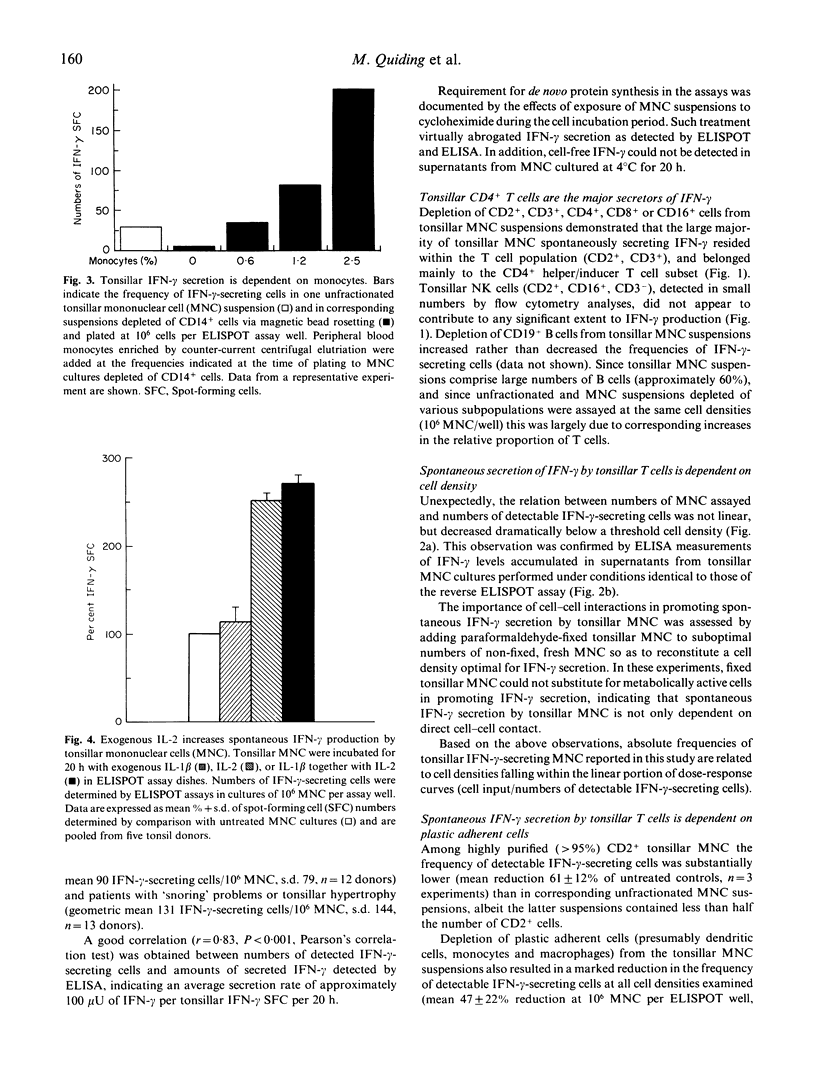
The frequency of mononuclear cells (MNC) spontaneously secreting interferon-gamma (IFN-gamma) has been examined in freshly isolated cell suspensions from human palatine tonsils. Two-site reverse enzyme-linked immunospot (ELISPOT) analyses, involving short term (20 h) incubation of MNC in the absence of any added exogenous stimulus, revealed that tonsillar MNC suspensions contain exceptionally large numbers of cells secreting IFN-gamma. No significant differences were observed when comparing the frequency of IFN-gamma-producing cells between cell suspensions obtained from hyperplastic and tonsillitis specimens. Cell-sorting experiments disclosed that spontaneous tonsillar IFN-gamma production was essentially contributed by CD4+ T cells, and required the presence of accessory cells and/or soluble factors to be detected. Thus, depletion of plastic adherent cells or monocytes from the tonsillar MNC suspensions resulted in reduced numbers of detectable IFN-gamma-secreting cells. Addition of very small numbers of autologous monocytes restored spontaneous IFN-gamma production in tonsillar MNC cultures depleted of monocytes. Neutralization of endogenous IL-1 beta and IL-2, as well as blocking of the IL-2 receptor, also decreased IFN-gamma production from unfractionated tonsillar cells. Addition of exogenous IL-1 beta restored IFN-gamma production in cultures of tonsillar MNC depleted of plastic adherent cells. Furthermore, IL-1 beta synergized with IL-2 by tonsillar MNC depleted of plastic adherent cells. Furthermore, IL-1 beta synergized with IL-2 by increasing intracellular as well as cell-free levels of IFN-gamma in cultures of unfractionated tonsillar MNC. This study further establishes that the tonsils are highly active immunological organs containing large numbers of T cells spontaneously producing IFN-gamma whose detection is contingent upon the presence of functional accessory cells. It also demonstrates that concomitant production of IL-1 beta and IL-2 occurs in tonsils and is necessary to maintain ongoing synthesis and extracellular accumulation of IFN-gamma in these organs.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson G., Ekre H. P., Alm G., Perlmann P. Monoclonal antibody two-site ELISA for human IFN-gamma. Adaptation for determinations in human serum or plasma. J Immunol Methods. 1989 Dec 20;125(1-2):89–96. doi: 10.1016/0022-1759(89)90081-1. [DOI] [PubMed] [Google Scholar]
- Bernstein J. M., Scheeren R., Schoenfeld E., Albini B. The distribution of immunocompetent cells in the compartments of the palatine tonsils in bacterial and viral infections of the upper respiratory tract. Acta Otolaryngol Suppl. 1988;454:153–162. doi: 10.3109/00016488809125019. [DOI] [PubMed] [Google Scholar]
- Beutler B., Cerami A. Cachectin: more than a tumor necrosis factor. N Engl J Med. 1987 Feb 12;316(7):379–385. doi: 10.1056/NEJM198702123160705. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P., Surjan L., Jr, Berdal P. Immunoglobulin-producing cells in clinically normal, hyperplastic and inflamed human palatine tonsils. Acta Otolaryngol Suppl. 1979;360:211–215. doi: 10.3109/00016487809123519. [DOI] [PubMed] [Google Scholar]
- Chan S. H., Perussia B., Gupta J. W., Kobayashi M., Pospísil M., Young H. A., Wolf S. F., Young D., Clark S. C., Trinchieri G. Induction of interferon gamma production by natural killer cell stimulatory factor: characterization of the responder cells and synergy with other inducers. J Exp Med. 1991 Apr 1;173(4):869–879. doi: 10.1084/jem.173.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czerkinsky C., Andersson G., Ekre H. P., Nilsson L. A., Klareskog L., Ouchterlony O. Reverse ELISPOT assay for clonal analysis of cytokine production. I. Enumeration of gamma-interferon-secreting cells. J Immunol Methods. 1988 May 25;110(1):29–36. doi: 10.1016/0022-1759(88)90079-8. [DOI] [PubMed] [Google Scholar]
- Ehlers S., Smith K. A. Differentiation of T cell lymphokine gene expression: the in vitro acquisition of T cell memory. J Exp Med. 1991 Jan 1;173(1):25–36. doi: 10.1084/jem.173.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar W. L., Birchenall-Sparks M. C., Young H. B. Interleukin 2 induction of interferon-gamma mRNA synthesis. J Immunol. 1986 Dec 15;137(12):3836–3840. [PubMed] [Google Scholar]
- Ferrua B., Aussel C., Fehlmann M. Human interleukin 2. Detection at the picomolar level by sandwich enzyme immunoassay. J Immunol Methods. 1987 Mar 12;97(2):215–220. doi: 10.1016/0022-1759(87)90462-5. [DOI] [PubMed] [Google Scholar]
- Ferrua B., Becker P., Schaffar L., Shaw A., Fehlmann M. Detection of human IL-1 alpha and IL-1 beta at the subpicomolar level by colorimetric sandwich enzyme immunoassay. J Immunol Methods. 1988 Nov 10;114(1-2):41–48. doi: 10.1016/0022-1759(88)90151-2. [DOI] [PubMed] [Google Scholar]
- Hellstrand K., Hermodsson S. Monocyte-mediated suppression of IL-2-induced NK-cell activation. Regulation by 5-HT1A-type serotonin receptors. Scand J Immunol. 1990 Aug;32(2):183–192. doi: 10.1111/j.1365-3083.1990.tb02908.x. [DOI] [PubMed] [Google Scholar]
- Holmgren J., Fryklund J., Larsson H. Gamma-interferon-mediated down-regulation of electrolyte secretion by intestinal epithelial cells: a local immune mechanism? Scand J Immunol. 1989 Oct;30(4):499–503. doi: 10.1111/j.1365-3083.1989.tb02456.x. [DOI] [PubMed] [Google Scholar]
- Howie A. J. Scanning and transmission electron microscopy on the epithelium of human palatine tonsils. J Pathol. 1980 Feb;130(2):91–98. doi: 10.1002/path.1711300205. [DOI] [PubMed] [Google Scholar]
- Kelly C. D., Russo C. M., Rubin B. Y., Murray H. W. Antigen-stimulated human interferon-gamma generation: role of accessory cells and their expressed or secreted products. Clin Exp Immunol. 1989 Sep;77(3):397–402. [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M., Fitz L., Ryan M., Hewick R. M., Clark S. C., Chan S., Loudon R., Sherman F., Perussia B., Trinchieri G. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J Exp Med. 1989 Sep 1;170(3):827–845. doi: 10.1084/jem.170.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le J., Lin J. X., Henriksen-DeStefano D., Vilcek J. Bacterial lipopolysaccharide-induced interferon-gamma production: roles of interleukin 1 and interleukin 2. J Immunol. 1986 Jun 15;136(12):4525–4530. [PubMed] [Google Scholar]
- Lieberman B. Y., Fiocchi C., Youngman K. R., Sapatnekar W. K., Proffitt M. R. Interferon gamma production by human intestinal mucosal mononuclear cells. Decreased levels in inflammatory bowel disease. Dig Dis Sci. 1988 Oct;33(10):1297–1304. doi: 10.1007/BF01536683. [DOI] [PubMed] [Google Scholar]
- Madara J. L., Stafford J. Interferon-gamma directly affects barrier function of cultured intestinal epithelial monolayers. J Clin Invest. 1989 Feb;83(2):724–727. doi: 10.1172/JCI113938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadal D., Albini B., Chen C. Y., Schläpfer E., Bernstein J. M., Ogra P. L. Distribution and engraftment patterns of human tonsillar mononuclear cells and immunoglobulin-secreting cells in mice with severe combined immunodeficiency: role of the Epstein-Barr virus. Int Arch Allergy Appl Immunol. 1991;95(4):341–351. doi: 10.1159/000235471. [DOI] [PubMed] [Google Scholar]
- Nadal D., Albini B., Schläpfer E., Chen C., Brodsky L., Ogra P. L. Tissue distribution of mucosal antibody-producing cells specific for respiratory syncytial virus in severe combined immune deficiency (SCID) mice engrafted with human tonsils. Clin Exp Immunol. 1991 Sep;85(3):358–364. doi: 10.1111/j.1365-2249.1991.tb05732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naiem M., Gerdes J., Abdulaziz Z., Stein H., Mason D. Y. Production of a monoclonal antibody reactive with human dendritic reticulum cells and its use in the immunohistological analysis of lymphoid tissue. J Clin Pathol. 1983 Feb;36(2):167–175. doi: 10.1136/jcp.36.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto Y., Brodsky L., Bernstein J. M., Ogra P. L. Characteristics of in vitro production of mucosal antibody to respiratory syncytial virus in tonsillar tissue lymphocytes. Clin Immunol Immunopathol. 1988 Nov;49(2):299–307. doi: 10.1016/0090-1229(88)90120-1. [DOI] [PubMed] [Google Scholar]
- Paganelli R., Levinsky R. J. Differences in specific antibody responses of human tonsillar cells to an oral and a parenteral antigen. Scand J Immunol. 1981 Oct;14(4):353–358. doi: 10.1111/j.1365-3083.1981.tb00575.x. [DOI] [PubMed] [Google Scholar]
- Parmentier H. K., van der Linden J. A., Krijnen J., van Wichen D. F., Rademakers L. H., Bloem A. C., Schuurman H. J. Human follicular dendritic cells: isolation and characteristics in situ and in suspension. Scand J Immunol. 1991 Apr;33(4):441–452. doi: 10.1111/j.1365-3083.1991.tb01793.x. [DOI] [PubMed] [Google Scholar]
- Pestka S., Langer J. A., Zoon K. C., Samuel C. E. Interferons and their actions. Annu Rev Biochem. 1987;56:727–777. doi: 10.1146/annurev.bi.56.070187.003455. [DOI] [PubMed] [Google Scholar]
- Platts-Mills T. A., Ishizaka K. IgA and IgA diphtheria antitoxin responses from human tonsil lymphocytes. J Immunol. 1975 Mar;114(3):1058–1064. [PubMed] [Google Scholar]
- Quiding M., Nordström I., Kilander A., Andersson G., Hanson L. A., Holmgren J., Czerkinsky C. Intestinal immune responses in humans. Oral cholera vaccination induces strong intestinal antibody responses and interferon-gamma production and evokes local immunological memory. J Clin Invest. 1991 Jul;88(1):143–148. doi: 10.1172/JCI115270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reem G. H., Yeh N. H. Interleukin 2 regulates expression of its receptor and synthesis of gamma interferon by human T lymphocytes. Science. 1984 Jul 27;225(4660):429–430. doi: 10.1126/science.6429853. [DOI] [PubMed] [Google Scholar]
- Saxon A., Feldhaus J., Robins R. A. Single step separation of human T and B cells using AET treated srbc rosettes. J Immunol Methods. 1976;12(3-4):285–288. doi: 10.1016/0022-1759(76)90050-8. [DOI] [PubMed] [Google Scholar]
- Siggens K. W., Wilkinson M. F., Boseley P. G., Slocombe P. M., Cowling G., Morris A. G. Differences in the expression of the human interferon-gamma gene in fresh lymphocytes and cultured lymphoblasts. Biochem Biophys Res Commun. 1984 Feb 29;119(1):157–162. doi: 10.1016/0006-291x(84)91632-2. [DOI] [PubMed] [Google Scholar]
- Sollid L. M., Kvale D., Brandtzaeg P., Markussen G., Thorsby E. Interferon-gamma enhances expression of secretory component, the epithelial receptor for polymeric immunoglobulins. J Immunol. 1987 Jun 15;138(12):4303–4306. [PubMed] [Google Scholar]
- Van Snick J. Interleukin-6: an overview. Annu Rev Immunol. 1990;8:253–278. doi: 10.1146/annurev.iy.08.040190.001345. [DOI] [PubMed] [Google Scholar]
- Versteegen J. M., Logtenberg T., Ballieux R. E. Enumeration of IFN-gamma-producing human lymphocytes by spot-ELISA. A method to detect lymphokine-producing lymphocytes at the single-cell level. J Immunol Methods. 1988 Jun 28;111(1):25–29. doi: 10.1016/0022-1759(88)90055-5. [DOI] [PubMed] [Google Scholar]
- Vilcek J., Henriksen-Destefano D., Siegel D., Klion A., Robb R. J., Le J. Regulation of IFN-gamma induction in human peripheral blood cells by exogenous and endogenously produced interleukin 2. J Immunol. 1985 Sep;135(3):1851–1856. [PubMed] [Google Scholar]


