Abstract
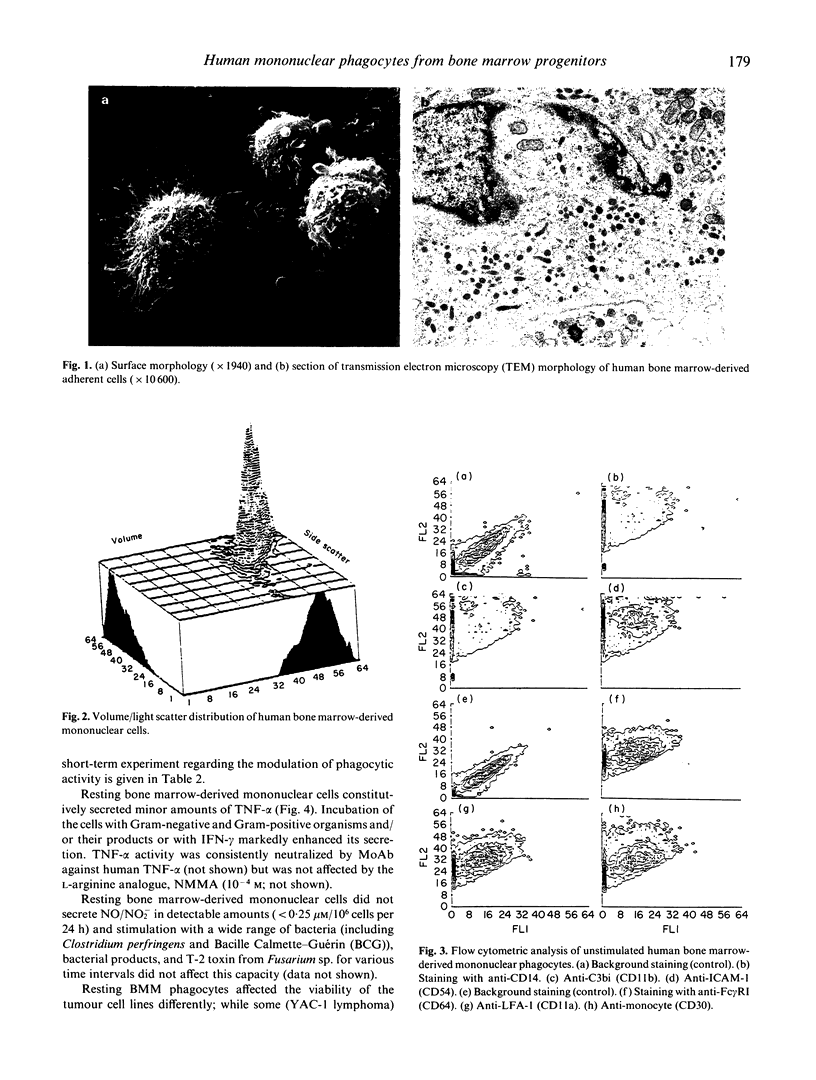
After 3-4 weeks culture of human bone marrow cells in medium supplemented with IL-3, macrophage- (M-CSF), and granulocyte-macrophage colony-stimulating factor (GM-CSF), the firmly adherent cells exhibited the morphologic features of mononuclear phagocytes and were strongly esterase-positive. Flow cytometric analysis revealed a rather homogeneous cell population with marked autofluorescence; the large majority of the cells expressed CD14, CD11a, b, and c, Fc receptors for IgG, Fc gamma RI, II, and III, and HLA class II molecules. Interferon-gamma (IFN-gamma), bacteria, and bacterial products modulated expression of some of the surface markers, induced and/or enhanced respiratory burst, phagocytic activity, secretion of tumour necrosis factor, and tumouricidal activity; in contrast, these cells were not able to generate reactive nitrogen intermediates.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreoni C., Moreau I., Rigal D. Long-term culture of human bone marrow. I. characterization of adherent cells in flow cytometry. Exp Hematol. 1990 Jun;18(5):431–437. [PubMed] [Google Scholar]
- Drach J., Gattringer C., Glassl H., Schwarting R., Stein H., Huber H. Simultaneous flow cytometric analysis of surface markers and nuclear Ki-67 antigen in leukemia and lymphoma. Cytometry. 1989 Nov;10(6):743–749. doi: 10.1002/cyto.990100612. [DOI] [PubMed] [Google Scholar]
- Dransfield I., Corcoran D., Partridge L. J., Hogg N., Burton D. R. Comparison of human monocytes isolated by elutriation and adherence suggests that heterogeneity may reflect a continuum of maturation/activation states. Immunology. 1988 Mar;63(3):491–498. [PMC free article] [PubMed] [Google Scholar]
- Gartner S., Kaplan H. S. Long-term culture of human bone marrow cells. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4756–4759. doi: 10.1073/pnas.77.8.4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocking W. G., Golde D. W. Long-term human bone marrow cultures. Blood. 1980 Jul;56(1):118–124. [PubMed] [Google Scholar]
- Hogg N. Human mononuclear phagocyte molecules and the use of monoclonal antibodies in their detection. Clin Exp Immunol. 1987 Sep;69(3):687–694. [PMC free article] [PubMed] [Google Scholar]
- Keller R., Gehri R., Keist R., Huf E., Kayser F. H. The interaction of macrophages and bacteria: a comparative study of the induction of tumoricidal activity and of reactive nitrogen intermediates. Cell Immunol. 1991 Apr 15;134(1):249–256. doi: 10.1016/0008-8749(91)90348-f. [DOI] [PubMed] [Google Scholar]
- Keller R., Gehri R., Keist R. The interaction of macrophages and bacteria: Escherichia coli species, bacterial lipopolysaccharide, and lipid A differ in their ability to induce tumoricidal activity and the secretion of reactive nitrogen intermediates in macrophages. Cell Immunol. 1992 Apr 15;141(1):47–58. doi: 10.1016/0008-8749(92)90126-a. [DOI] [PubMed] [Google Scholar]
- Keller R., Gustafson J. E., Keist R. The macrophage response to bacteria. Modulation of macrophage functional activity by peptidoglycan from Moraxella (Branhamella) catarrhalis. Clin Exp Immunol. 1992 Sep;89(3):384–389. doi: 10.1111/j.1365-2249.1992.tb06967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller R., Joller P. W., Keist R. Surface phenotype of rat bone marrow-derived mononuclear phagocytes. Cell Immunol. 1989 Apr 15;120(1):277–285. doi: 10.1016/0008-8749(89)90195-0. [DOI] [PubMed] [Google Scholar]
- Keller R., Keist R., Bazin H., Joller P., Van der Meide P. H. Binding of monomeric immunoglobulins by bone marrow-derived mononuclear phagocytes; its modulation by interferon-gamma. Eur J Immunol. 1990 Sep;20(9):2137–2140. doi: 10.1002/eji.1830200937. [DOI] [PubMed] [Google Scholar]
- Keller R., Keist R. Tumor-promoting phorbol esters induce macrophage differentiation from bone marrow precursors. Exp Cell Biol. 1982;50(5):255–265. doi: 10.1159/000163154. [DOI] [PubMed] [Google Scholar]
- Keller R., Keist R., Van der Meide P. H., Groscurth P., Aguet M., Leist T. P. Induction, maintenance, and reinduction of tumoricidal activity in bone marrow-derived mononuclear phagocytes by Corynebacterium parvum. Evidence for the involvement of a T cell- and interferon-gamma-independent pathway of macrophage activation. J Immunol. 1987 Apr 1;138(7):2366–2371. [PubMed] [Google Scholar]
- Keller R., Keist R., Wechsler A., Leist T. P., van der Meide P. H. Mechanisms of macrophage-mediated tumor cell killing: a comparative analysis of the roles of reactive nitrogen intermediates and tumor necrosis factor. Int J Cancer. 1990 Oct 15;46(4):682–686. doi: 10.1002/ijc.2910460422. [DOI] [PubMed] [Google Scholar]
- Keller R., Keist R., van der Meide P. H. Modulation of tumoricidal activity, induced in bone-marrow-derived mononuclear phagocytes by interferon gamma or Corynebacterium parvum, by interferon beta, tumor necrosis factor, prostaglandin E2, and transforming growth factor beta. Int J Cancer. 1991 Nov 11;49(5):796–800. doi: 10.1002/ijc.2910490526. [DOI] [PubMed] [Google Scholar]
- Klostergaard J., Leroux M. E. L-arginine independent macrophage tumor cytotoxicity. Biochem Biophys Res Commun. 1989 Dec 29;165(3):1262–1266. doi: 10.1016/0006-291x(89)92738-1. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Philip R. Cytolysis of tumor necrosis factor (TNF)-resistant tumor targets. Differential cytotoxicity of monocytes activated by the interferons, IL-2, and TNF. J Immunol. 1988 Feb 15;140(4):1345–1349. [PubMed] [Google Scholar]
- Shibata T., Inoue S. Mature T cells are part of adherent cells in human long-term bone marrow cultures. Exp Hematol. 1986 Mar;14(3):234–240. [PubMed] [Google Scholar]
- Stanley E. R., Heard P. M. Factors regulating macrophage production and growth. Purification and some properties of the colony stimulating factor from medium conditioned by mouse L cells. J Biol Chem. 1977 Jun 25;252(12):4305–4312. [PubMed] [Google Scholar]
- Strobel E. S., Gay R. E., Greenberg P. L. Characterization of the in vitro stromal microenvironment of human bone marrow. Int J Cell Cloning. 1986 Sep;4(5):341–356. doi: 10.1002/stem.5530040506. [DOI] [PubMed] [Google Scholar]
- Witsell A. L., Schook L. B. Macrophage heterogeneity occurs through a developmental mechanism. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1963–1967. doi: 10.1073/pnas.88.5.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]



