Abstract
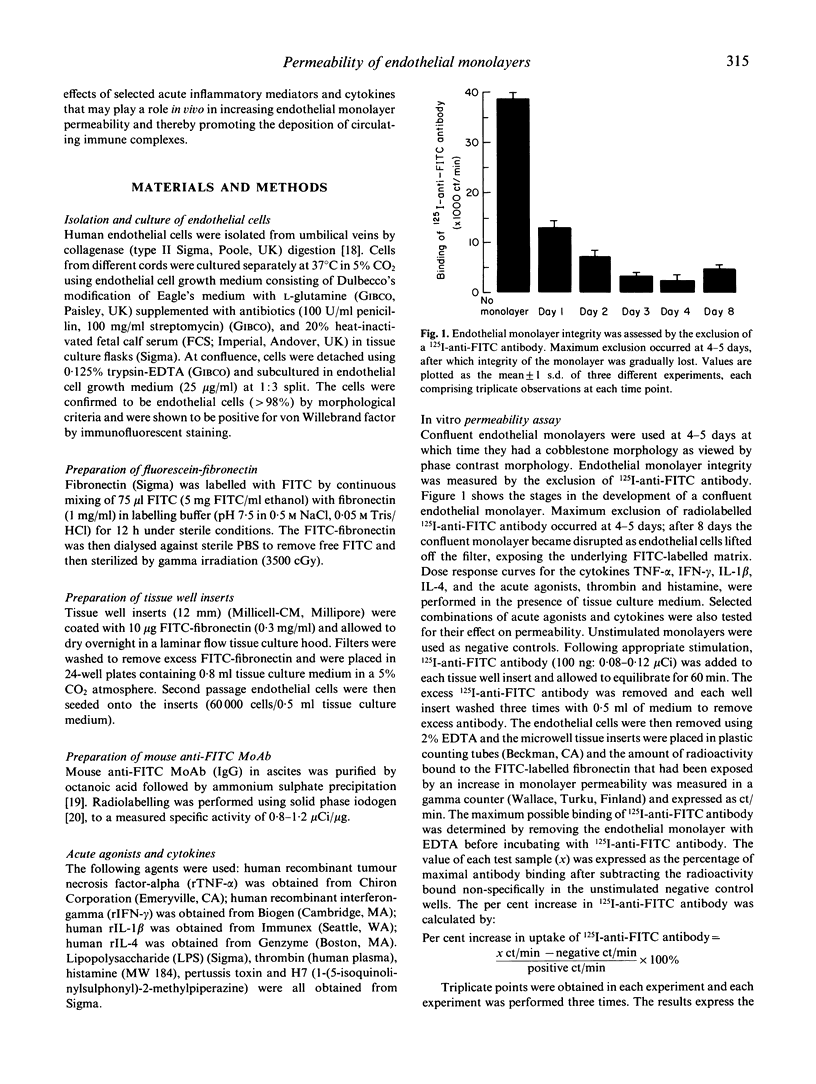
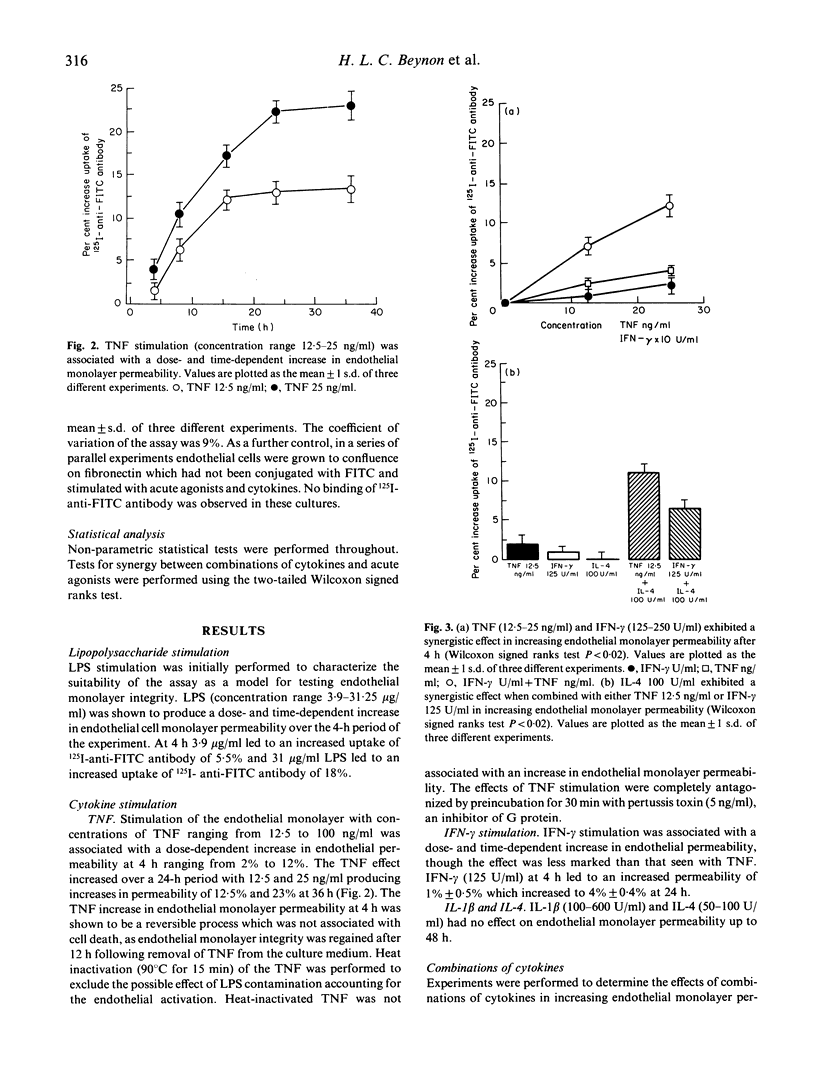
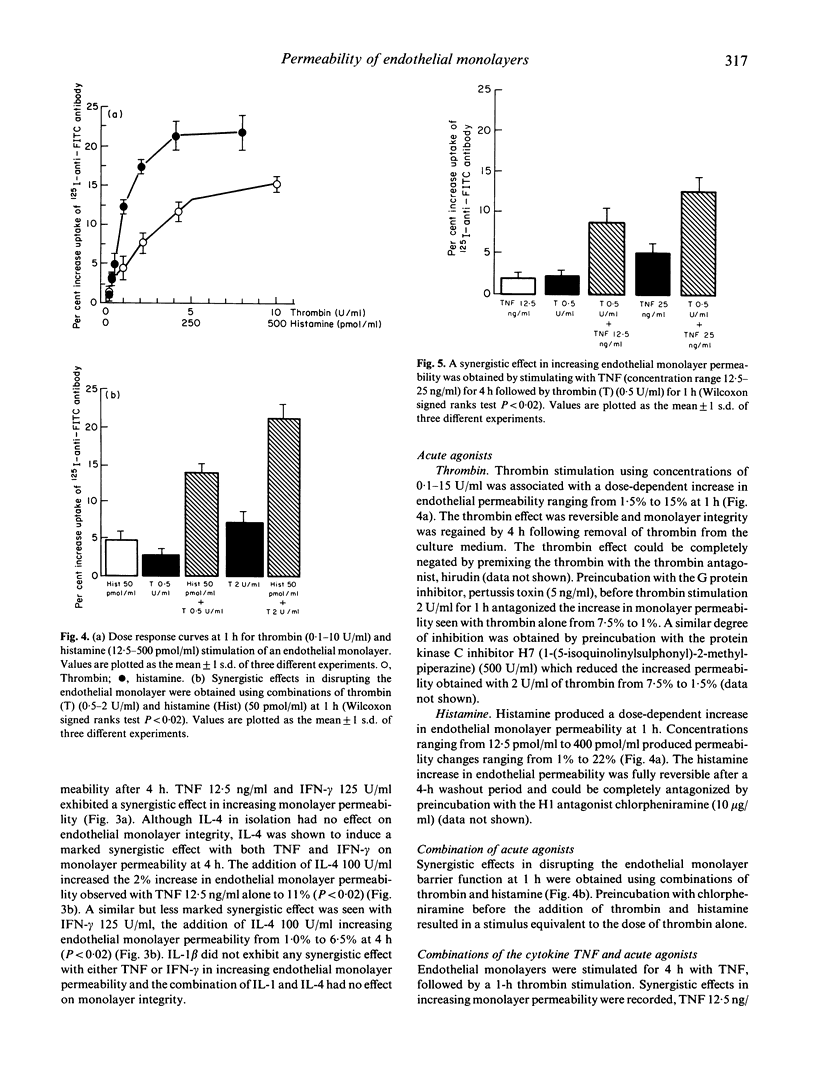
The deposition of circulating immune reactants in blood vessels, an important event in the pathogenesis of certain types of vasculitis, requires an increase in permeability in the endothelial monolayer. An in vitro model to examine the integrity of endothelial cell monolayers and their response to inflammatory mediators has been developed. Human umbilical vein endothelial cells were grown to confluence on an FITC-labelled matrix and monolayer integrity was assessed by the exclusion of a 125I-anti-FITC antibody. Alteration in endothelial monolayer permeability was associated with an increase in uptake of 125I-anti-FITC antibody, expressed as a percentage of the maximal uptake of antibody on to FITC-matrix from which endothelial cells had been stripped. We determined the effects on endothelial monolayer permeability of acute agonists (thrombin and histamine), cytokines (tumour necrosis factor-alpha (TNF-alpha), interferon-gamma (IFN-gamma), IL-1 and IL-4) and combinations of acute agonists and cytokines. Addition of thrombin in concentrations ranging from 0.5 to 15 U/ml led to an increased uptake of 125I-anti-FITC antibody from 2% to 15% relative to unstimulated endothelium. For other agonists and cytokines the increases in permeability were: (i) histamine (50-400 pmol/ml) increased uptake 5-22%; (ii) TNF (12.5-100 ng/ml) increased uptake 2-12%; (iii) IFN-gamma (125-250 U/ml) increased uptake 1.5-3%. IL-1 beta (50-100 U/ml) and IL-4 (50-100 U/ml) had no effect. Synergistic interactions on endothelial monolayer permeability were seen with the following combinations: (i) IL-4 (100 U/ml) and TNF (12.5 ng/ml) uptake 11%; (ii) IL-4 (100 U/ml) and IFN-gamma (125 U/ml) uptake 6.5%; (iii) TNF (12.5 ng/ml) and IFN-gamma (125 ng/ml) uptake 7%; (iv) thrombin (0.5 U/ml) and histamine (50 pmol/ml) uptake 13.5%; and (v) TNF (12.5 ng/ml) and thrombin (0.5 U/ml) uptake 8.5%. These observations suggest that interactions between cytokines and acute inflammatory mediators such as thrombin and histamine may be important in determining whether immune complexes are deposited in vessel walls. This model system may now be useful for the further investigation in vitro of the mechanisms involved in the pathogenesis of immune complex-mediated vascular damage.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baucke R. B., Spear P. G. Membrane proteins specified by herpes simplex viruses. V. Identification of an Fc-binding glycoprotein. J Virol. 1979 Dec;32(3):779–789. doi: 10.1128/jvi.32.3.779-789.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boiadjieva S., Hallberg C., Högström M., Busch C. Methods in laboratory investigation. Exclusion of trypan blue from microcarriers by endothelial cells: an in vitro barrier function test. Lab Invest. 1984 Feb;50(2):239–246. [PubMed] [Google Scholar]
- Bottaro D., Shepro D., Peterson S., Hechtman H. B. Serotonin, norepinephrine, and histamine mediation of endothelial cell barrier function in vitro. J Cell Physiol. 1986 Aug;128(2):189–194. doi: 10.1002/jcp.1041280208. [DOI] [PubMed] [Google Scholar]
- Brett J., Gerlach H., Nawroth P., Steinberg S., Godman G., Stern D. Tumor necrosis factor/cachectin increases permeability of endothelial cell monolayers by a mechanism involving regulatory G proteins. J Exp Med. 1989 Jun 1;169(6):1977–1991. doi: 10.1084/jem.169.6.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COCHRANE C. G., WEIGLE W. O., DIXON F. J. The role of polymorphonuclear leukocytes in the initiation and cessation of the Arthus vasculitis. J Exp Med. 1959 Sep 1;110:481–494. doi: 10.1084/jem.110.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cines D. B., Lyss A. P., Bina M., Corkey R., Kefalides N. A., Friedman H. M. Fc and C3 receptors induced by herpes simplex virus on cultured human endothelial cells. J Clin Invest. 1982 Jan;69(1):123–128. doi: 10.1172/JCI110422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane C. G., Koffler D. Immune complex disease in experimental animals and man. Adv Immunol. 1973;16(0):185–264. doi: 10.1016/s0065-2776(08)60298-9. [DOI] [PubMed] [Google Scholar]
- Cream J. J. Immunofluorescent studies of the skin in cryoglobulinaemic vasculitis. Br J Dermatol. 1971 Jan;84(1):48–53. doi: 10.1111/j.1365-2133.1971.tb14196.x. [DOI] [PubMed] [Google Scholar]
- Daha M. R., Miltenburg A. M., Hiemstra P. S., Klar-Mohamad N., Van Es L. A., Van Hinsbergh V. W. The complement subcomponent C1q mediates binding of immune complexes and aggregates to endothelial cells in vitro. Eur J Immunol. 1988 May;18(5):783–787. doi: 10.1002/eji.1830180519. [DOI] [PubMed] [Google Scholar]
- Del Vecchio P. J., Siflinger-Birnboim A., Shepard J. M., Bizios R., Cooper J. A., Malik A. B. Endothelial monolayer permeability to macromolecules. Fed Proc. 1987 Jun;46(8):2511–2515. [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Garcia J. G., Siflinger-Birnboim A., Bizios R., Del Vecchio P. J., Fenton J. W., 2nd, Malik A. B. Thrombin-induced increase in albumin permeability across the endothelium. J Cell Physiol. 1986 Jul;128(1):96–104. doi: 10.1002/jcp.1041280115. [DOI] [PubMed] [Google Scholar]
- Harlan J. M., Harker L. A., Reidy M. A., Gajdusek C. M., Schwartz S. M., Striker G. E. Lipopolysaccharide-mediated bovine endothelial cell injury in vitro. Lab Invest. 1983 Mar;48(3):269–274. [PubMed] [Google Scholar]
- Haselton F. R., Mueller S. N., Howell R. E., Levine E. M., Fishman A. P. Chromatographic demonstration of reversible changes in endothelial permeability. J Appl Physiol (1985) 1989 Nov;67(5):2032–2048. doi: 10.1152/jappl.1989.67.5.2032. [DOI] [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KNIKER W. T., COCHRANE C. G. PATHOGENIC FACTORS IN VASULAR LESIONS OF EXPERIMENTAL SERUM SICKNESS. J Exp Med. 1965 Jul 1;122:83–98. doi: 10.1084/jem.122.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killackey J. J., Johnston M. G., Movat H. Z. Increased permeability of microcarrier-cultured endothelial monolayers in response to histamine and thrombin. A model for the in vitro study of increased vasopermeability. Am J Pathol. 1986 Jan;122(1):50–61. [PMC free article] [PubMed] [Google Scholar]
- Laposata M., Dovnarsky D. K., Shin H. S. Thrombin-induced gap formation in confluent endothelial cell monolayers in vitro. Blood. 1983 Sep;62(3):549–556. [PubMed] [Google Scholar]
- Linder E. Binding of C1q and complement activation by vascular endothelium. J Immunol. 1981 Feb;126(2):648–658. [PubMed] [Google Scholar]
- Lloyd W., Schur P. H. Immune complexes, complement, and anti-DNA in exacerbations of systemic lupus erythematosus (SLE). Medicine (Baltimore) 1981 May;60(3):208–217. doi: 10.1097/00005792-198105000-00004. [DOI] [PubMed] [Google Scholar]
- Majno G., Shea S. M., Leventhal M. Endothelial contraction induced by histamine-type mediators: an electron microscopic study. J Cell Biol. 1969 Sep;42(3):647–672. doi: 10.1083/jcb.42.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyrick B. O., Ryan U. S., Brigham K. L. Direct effects of E coli endotoxin on structure and permeability of pulmonary endothelial monolayers and the endothelial layer of intimal explants. Am J Pathol. 1986 Jan;122(1):140–151. [PMC free article] [PubMed] [Google Scholar]
- Ryan U. S., Schultz D. R., Ruan J. W. Fc and C3b receptors on pulmonary endothelial cells: induction by injury. Science. 1981 Oct 30;214(4520):557–558. doi: 10.1126/science.6270789. [DOI] [PubMed] [Google Scholar]
- Shusterman N., London W. T. Hepatitis B and immune-complex disease. N Engl J Med. 1984 Jan 5;310(1):43–46. doi: 10.1056/NEJM198401053100110. [DOI] [PubMed] [Google Scholar]
- Steinbuch M., Audran R. The isolation of IgG from mammalian sera with the aid of caprylic acid. Arch Biochem Biophys. 1969 Nov;134(2):279–284. doi: 10.1016/0003-9861(69)90285-9. [DOI] [PubMed] [Google Scholar]
- Stolpen A. H., Guinan E. C., Fiers W., Pober J. S. Recombinant tumor necrosis factor and immune interferon act singly and in combination to reorganize human vascular endothelial cell monolayers. Am J Pathol. 1986 Apr;123(1):16–24. [PMC free article] [PubMed] [Google Scholar]
- Thornhill M. H., Haskard D. O. IL-4 regulates endothelial cell activation by IL-1, tumor necrosis factor, or IFN-gamma. J Immunol. 1990 Aug 1;145(3):865–872. [PubMed] [Google Scholar]
- WARD P. A., COCHRANE C. G., MUELLER-EBERHARD H. J. THE ROLE OF SERUM COMPLEMENT IN CHEMOTAXIS OF LEUKOCYTES IN VITRO. J Exp Med. 1965 Aug 1;122:327–346. doi: 10.1084/jem.122.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S. C., Schultz D. R., Ryan U. S. Receptor-mediated binding of C1q on pulmonary endothelial cells. Tissue Cell. 1986;18(1):13–18. doi: 10.1016/0040-8166(86)90003-0. [DOI] [PubMed] [Google Scholar]


