Abstract
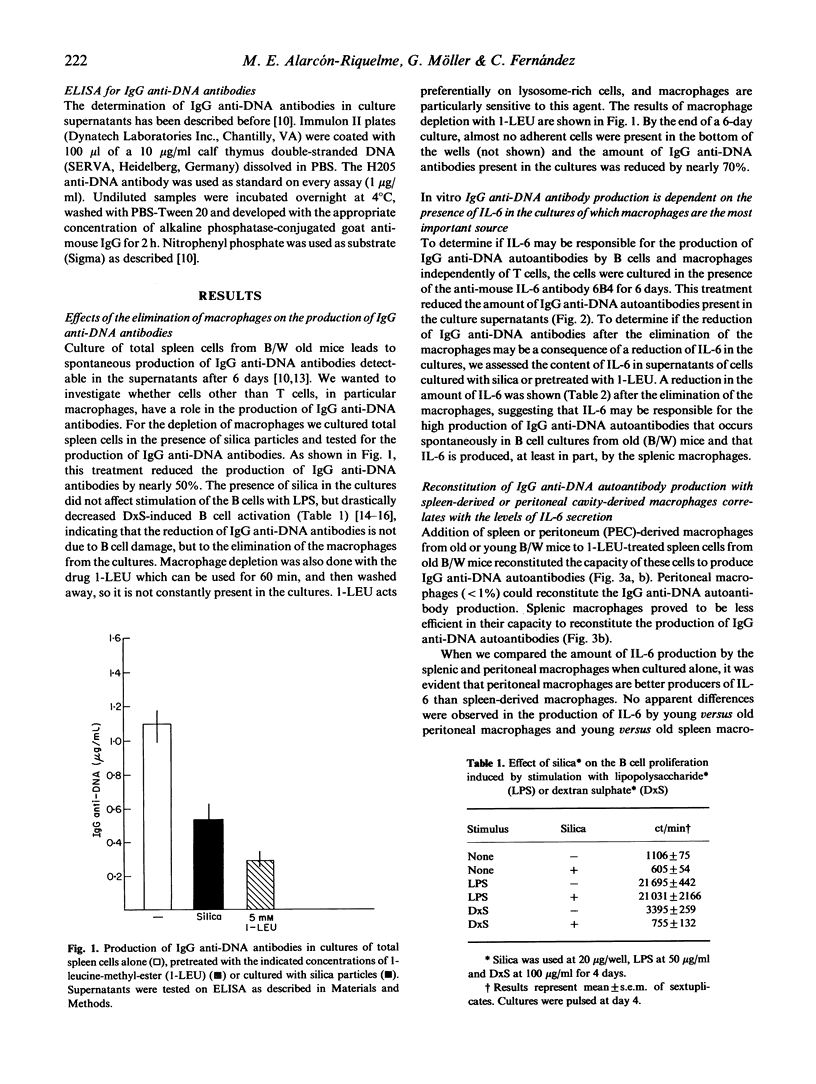
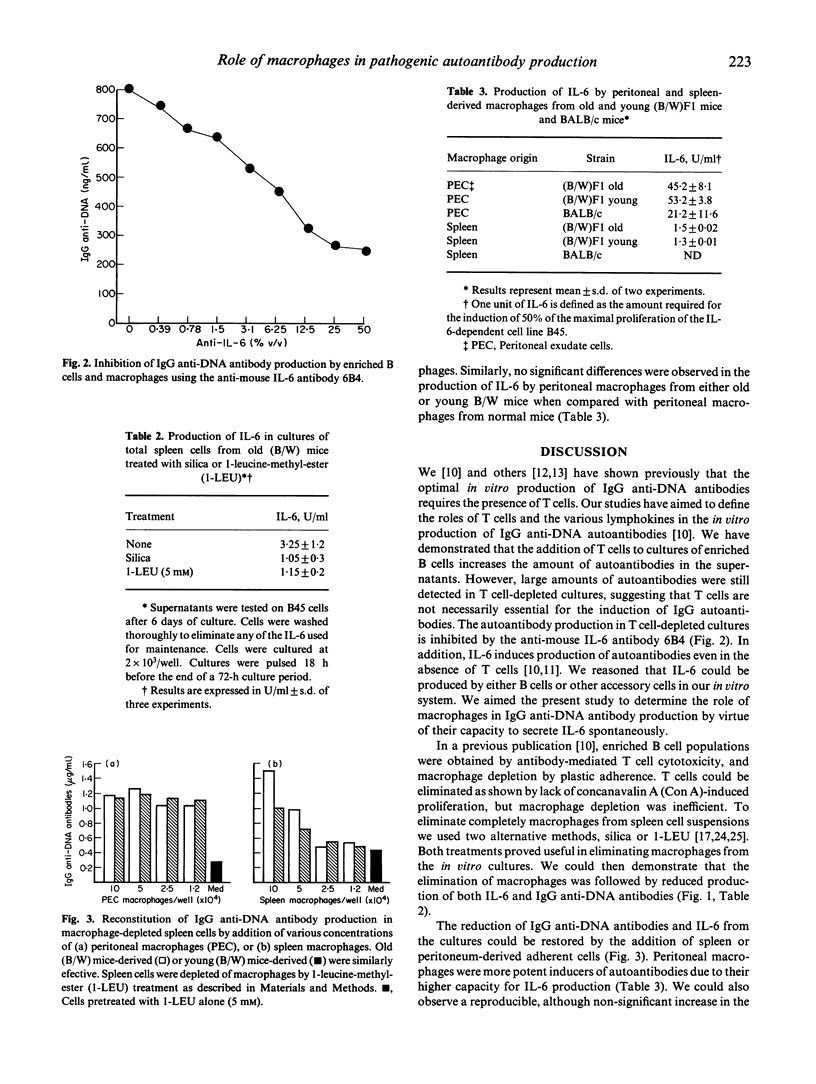
We have studied the role of macrophages in the production of IgG anti-DNA autoantibodies by (NZB x NZW)F1 mice (B/W). One of the main features of the systemic lupus erythematosus (SLE)-like disease that affects these mice, is the presence of circulating IgG autoantibodies and immune complexes, which lead to renal failure and death by the age of 8-9 months. IgG autoantibodies are produced without in vitro stimulation by total spleen cells from these mice when they reach the age of 6 months. We have demonstrated that IL-6 increases the production of IgG autoantibodies in cultures of splenic purified B cells from the old B/W mice. The aim of this study was to show the involvement of macrophages in the production of IL-6 and consequently in the production of IgG anti-DNA antibodies in vitro. We show that elimination of the macrophages by different treatments led to reduction of the content of IL-6 in the supernatants as well as of IgG anti-DNA autoantibodies. Addition of fresh, splenic or peritoneal macrophages restored the production of autoantibodies in macrophage-depleted cultures from old B/W mice. There were no differences in the capacity of IL-6 production between macrophages from old or young B/W mice, but an important difference was observed between peritoneal and splenic macrophages, where the former produced much higher levels of IL-6, and consequently were more potent inducers of IgG autoantibodies. The present results reinforce the role of macrophages and IL-6 in the production of IgG anti-DNA autoantibodies in B/W mice. The implications of these results in the pathogenesis of the disease are discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alarcón-Riquelme M. E., Möller G., Fernández C. Age-dependent responsiveness to interleukin-6 in B lymphocytes from a systemic lupus erythematosus-prone (NZB x NZW)F1 hybrid. Clin Immunol Immunopathol. 1992 Mar;62(3):264–269. doi: 10.1016/0090-1229(92)90101-s. [DOI] [PubMed] [Google Scholar]
- Allison A. C., Harington J. S., Birbeck M. An examination of the cytotoxic effects of silica on macrophages. J Exp Med. 1966 Aug 1;124(2):141–154. doi: 10.1084/jem.124.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergstedt-Lindqvist S., Fernandez C., Severinson E. A synergistic polyclonal response to dextran sulphate and lipopolysaccharide: immunoglobulin secretion and cell requirements. Scand J Immunol. 1981 May;15(5):439–448. doi: 10.1111/j.1365-3083.1982.tb00669.x. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A. Inflammatory cytokines: interleukin-1 and tumor necrosis factor as effector molecules in autoimmune diseases. Curr Opin Immunol. 1991 Dec;3(6):941–948. doi: 10.1016/s0952-7915(05)80018-4. [DOI] [PubMed] [Google Scholar]
- Henter J. I., Elinder G., Söder O., Hansson M., Andersson B., Andersson U. Hypercytokinemia in familial hemophagocytic lymphohistiocytosis. Blood. 1991 Dec 1;78(11):2918–2922. [PubMed] [Google Scholar]
- Hirano T. Interleukin-6 and its relation to inflammation and disease. Clin Immunol Immunopathol. 1992 Jan;62(1 Pt 2):S60–S65. doi: 10.1016/0090-1229(92)90042-m. [DOI] [PubMed] [Google Scholar]
- Hirano T., Matsuda T., Turner M., Miyasaka N., Buchan G., Tang B., Sato K., Shimizu M., Maini R., Feldmann M. Excessive production of interleukin 6/B cell stimulatory factor-2 in rheumatoid arthritis. Eur J Immunol. 1988 Nov;18(11):1797–1801. doi: 10.1002/eji.1830181122. [DOI] [PubMed] [Google Scholar]
- Hirano T., Taga T., Yasukawa K., Nakajima K., Nakano N., Takatsuki F., Shimizu M., Murashima A., Tsunasawa S., Sakiyama F. Human B-cell differentiation factor defined by an anti-peptide antibody and its possible role in autoantibody production. Proc Natl Acad Sci U S A. 1987 Jan;84(1):228–231. doi: 10.1073/pnas.84.1.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano M., Hirano T., Matsuda T., Taga T., Horii Y., Iwato K., Asaoku H., Tang B., Tanabe O., Tanaka H. Autocrine generation and requirement of BSF-2/IL-6 for human multiple myelomas. Nature. 1988 Mar 3;332(6159):83–85. doi: 10.1038/332083a0. [DOI] [PubMed] [Google Scholar]
- Kelso A. Cytokines: structure, function and synthesis. Curr Opin Immunol. 1989 Dec;2(2):215–225. doi: 10.1016/0952-7915(89)90191-x. [DOI] [PubMed] [Google Scholar]
- Levine J., Hartwell D., Beller D. I. Imbalanced cytokine production by macrophages from autoimmune-prone mice. Immunol Lett. 1991 Oct;30(2):183–192. doi: 10.1016/0165-2478(91)90023-4. [DOI] [PubMed] [Google Scholar]
- Marshak-Rothstein A., Fink P., Gridley T., Raulet D. H., Bevan M. J., Gefter M. L. Properties and applications of monoclonal antibodies directed against determinants of the Thy-1 locus. J Immunol. 1979 Jun;122(6):2491–2497. [PubMed] [Google Scholar]
- Mihara M., Fukui H., Koishihara Y., Saito M., Ohsugi Y. Immunologic abnormality in NZB/W F1 mice. Thymus-independent expansion of B cells responding to interleukin-6. Clin Exp Immunol. 1990 Dec;82(3):533–537. doi: 10.1111/j.1365-2249.1990.tb05485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray L., Martens C. The abnormal T lymphocytes in lpr mice transcribe interferon-gamma and tumor necrosis factor-alpha genes spontaneously in vivo. Eur J Immunol. 1989 Mar;19(3):563–565. doi: 10.1002/eji.1830190325. [DOI] [PubMed] [Google Scholar]
- Persson U. C., Hammarström L. L., Smith C. I. Macrophages are required for the dextran-sulfate induced activation of B lymphocytes. J Immunol. 1977 Sep;119(3):1138–1144. [PubMed] [Google Scholar]
- Rawle F. C., Shields J., Smith S. H., Iliescu V., Merkenschlager M., Beverley P. C., Callard R. E. B cell growth and differentiation induced by supernatants of transformed epithelial cell lines. Eur J Immunol. 1986 Aug;16(8):1017–1019. doi: 10.1002/eji.1830160825. [DOI] [PubMed] [Google Scholar]
- Reeves J. P., Decker R. S., Crie J. S., Wildenthal K. Intracellular disruption of rat heart lysosomes by leucine methyl ester: effects on protein degradation. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4426–4429. doi: 10.1073/pnas.78.7.4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reininger L., Radaszkiewicz T., Kosco M., Melchers F., Rolink A. G. Development of autoimmune disease in SCID mice populated with long-term "in vitro" proliferating (NZB x NZW)F1 pre-B cells. J Exp Med. 1992 Nov 1;176(5):1343–1353. doi: 10.1084/jem.176.5.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekigawa I., Ishida Y., Hirose S., Sato H., Shirai T. Cellular basis of in vitro anti-DNA antibody production: evidence for T cell dependence of IgG-class anti-DNA antibody synthesis in the (NZB X NZW)F1 hybrid. J Immunol. 1986 Feb 15;136(4):1247–1252. [PubMed] [Google Scholar]
- Sobel E. S., Katagiri T., Katagiri K., Morris S. C., Cohen P. L., Eisenberg R. A. An intrinsic B cell defect is required for the production of autoantibodies in the lpr model of murine systemic autoimmunity. J Exp Med. 1991 Jun 1;173(6):1441–1449. doi: 10.1084/jem.173.6.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang B., Matsuda T., Akira S., Nagata N., Ikehara S., Hirano T., Kishimoto T. Age-associated increase in interleukin 6 in MRL/lpr mice. Int Immunol. 1991 Mar;3(3):273–278. doi: 10.1093/intimm/3.3.273. [DOI] [PubMed] [Google Scholar]
- Theofilopoulos A. N., Dixon F. J. Murine models of systemic lupus erythematosus. Adv Immunol. 1985;37:269–390. doi: 10.1016/s0065-2776(08)60342-9. [DOI] [PubMed] [Google Scholar]
- Thiele D. L., Kurosaka M., Lipsky P. E. Phenotype of the accessory cell necessary for mitogen-stimulated T and B cell responses in human peripheral blood: delineation by its sensitivity to the lysosomotropic agent, L-leucine methyl ester. J Immunol. 1983 Nov;131(5):2282–2290. [PubMed] [Google Scholar]
- Thiele D. L., Lipsky P. E. Modulation of human natural killer cell function by L-leucine methyl ester: monocyte-dependent depletion from human peripheral blood mononuclear cells. J Immunol. 1985 Feb;134(2):786–793. [PubMed] [Google Scholar]
- Vink A., Coulie P. G., Wauters P., Nordan R. P., Van Snick J. B cell growth and differentiation activity of interleukin-HP1 and related murine plasmacytoma growth factors. Synergy with interleukin 1. Eur J Immunol. 1988 Apr;18(4):607–612. doi: 10.1002/eji.1830180418. [DOI] [PubMed] [Google Scholar]
- Waage A., Slupphaug G., Shalaby R. Glucocorticoids inhibit the production of IL6 from monocytes, endothelial cells and fibroblasts. Eur J Immunol. 1990 Nov;20(11):2439–2443. doi: 10.1002/eji.1830201112. [DOI] [PubMed] [Google Scholar]
- Watanabe-Fukunaga R., Brannan C. I., Copeland N. G., Jenkins N. A., Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992 Mar 26;356(6367):314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- Wilde D. B., Marrack P., Kappler J., Dialynas D. P., Fitch F. W. Evidence implicating L3T4 in class II MHC antigen reactivity; monoclonal antibody GK1.5 (anti-L3T4a) blocks class II MHC antigen-specific proliferation, release of lymphokines, and binding by cloned murine helper T lymphocyte lines. J Immunol. 1983 Nov;131(5):2178–2183. [PubMed] [Google Scholar]
- Yoshizaki K., Matsuda T., Nishimoto N., Kuritani T., Taeho L., Aozasa K., Nakahata T., Kawai H., Tagoh H., Komori T. Pathogenic significance of interleukin-6 (IL-6/BSF-2) in Castleman's disease. Blood. 1989 Sep;74(4):1360–1367. [PubMed] [Google Scholar]
- Zanker B., Walz G., Wieder K. J., Strom T. B. Evidence that glucocorticosteroids block expression of the human interleukin-6 gene by accessory cells. Transplantation. 1990 Jan;49(1):183–185. doi: 10.1097/00007890-199001000-00040. [DOI] [PubMed] [Google Scholar]


