Abstract
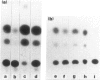
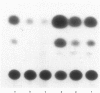
To test the hypothesis that CD8+ T cells inhibit viral replication at the level of cellular activation, an Epstein-Barr virus (EBV)-transformed cell line (FEc1) from a simian immunodeficiency virus (SIV)-seropositive sooty mangabey monkey was transfected with a human CD4 gene and shown to be replication-competent for HIV-1, HIV-2 and SIV. Utilizing a dual-chamber culture system, it was found that inhibition of viral replication can be mediated by a soluble factor. The FEc1 cell line was transiently transfected with an LTR-driven CAT reporter gene. It was found that autologous CD8+ T cells markedly inhibited CAT activity. Furthermore, co-transfection of the FEc1 cell line with an LTR-driven tat plasmid and LTR-CAT was able to quantitatively mitigate the suppressive effect. Thus, this inhibition appears to be directed at cellular mechanisms of viral transcription. Control transfections with an LTR-driven CAT plasmid with a mutation at the NFkB binding site yielded no CAT activity, suggesting that most viral replication as measured by CAT activity is dependent, to a large extent, upon cellularly derived NFkB binding proteins.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brinchmann J. E., Gaudernack G., Vartdal F. CD8+ T cells inhibit HIV replication in naturally infected CD4+ T cells. Evidence for a soluble inhibitor. J Immunol. 1990 Apr 15;144(8):2961–2966. [PubMed] [Google Scholar]
- Clavel F., Guyader M., Guétard D., Sallé M., Montagnier L., Alizon M. Molecular cloning and polymorphism of the human immune deficiency virus type 2. Nature. 1986 Dec 18;324(6098):691–695. doi: 10.1038/324691a0. [DOI] [PubMed] [Google Scholar]
- Fultz P. N., McClure H. M., Anderson D. C., Swenson R. B., Anand R., Srinivasan A. Isolation of a T-lymphotropic retrovirus from naturally infected sooty mangabey monkeys (Cercocebus atys). Proc Natl Acad Sci U S A. 1986 Jul;83(14):5286–5290. doi: 10.1073/pnas.83.14.5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene W. C., Böhnlein E., Ballard D. W. HIV-1, HTLV-1 and normal T-cell growth: transcriptional strategies and surprises. Immunol Today. 1989 Aug;10(8):272–278. doi: 10.1016/0167-5699(89)90141-2. [DOI] [PubMed] [Google Scholar]
- Greene W. C. Regulation of HIV-1 gene expression. Annu Rev Immunol. 1990;8:453–475. doi: 10.1146/annurev.iy.08.040190.002321. [DOI] [PubMed] [Google Scholar]
- Griffin G. E., Leung K., Folks T. M., Kunkel S., Nabel G. J. Activation of HIV gene expression during monocyte differentiation by induction of NF-kappa B. Nature. 1989 May 4;339(6219):70–73. doi: 10.1038/339070a0. [DOI] [PubMed] [Google Scholar]
- Herbomel P., Bourachot B., Yaniv M. Two distinct enhancers with different cell specificities coexist in the regulatory region of polyoma. Cell. 1984 Dec;39(3 Pt 2):653–662. doi: 10.1016/0092-8674(84)90472-0. [DOI] [PubMed] [Google Scholar]
- Kannagi M., Chalifoux L. V., Lord C. I., Letvin N. L. Suppression of simian immunodeficiency virus replication in vitro by CD8+ lymphocytes. J Immunol. 1988 Apr 1;140(7):2237–2242. [PubMed] [Google Scholar]
- Lane H. C., Depper J. M., Greene W. C., Whalen G., Waldmann T. A., Fauci A. S. Qualitative analysis of immune function in patients with the acquired immunodeficiency syndrome. Evidence for a selective defect in soluble antigen recognition. N Engl J Med. 1985 Jul 11;313(2):79–84. doi: 10.1056/NEJM198507113130204. [DOI] [PubMed] [Google Scholar]
- McClure H. M., Anderson D. C., Fultz P. N., Ansari A. A., Lockwood E., Brodie A. Spectrum of disease in macaque monkeys chronically infected with SIV/SMM. Vet Immunol Immunopathol. 1989 May;21(1):13–24. doi: 10.1016/0165-2427(89)90126-8. [DOI] [PubMed] [Google Scholar]
- Nabel G., Baltimore D. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature. 1987 Apr 16;326(6114):711–713. doi: 10.1038/326711a0. [DOI] [PubMed] [Google Scholar]
- Powell J. D., McClure H. M., Anderson D., Fultz P. N., Sell K. W., Ahmed-Ansari A. Phenotypic and functional differences in NK and LAK cells in the peripheral blood of sooty mangabeys and rhesus macaques. Cell Immunol. 1989 Nov;124(1):107–118. doi: 10.1016/0008-8749(89)90115-9. [DOI] [PubMed] [Google Scholar]
- Powell J. D., Yehuda-Cohen T., Villinger F., McClure H. M., Sell K. W., Ahmed-Ansari A. Inhibition of SIV/SMM replication in vitro by CD8+ cells from SIV/SMM infected seropositive clinically asymptomatic sooty mangabeys. J Med Primatol. 1990;19(3-4):239–249. [PubMed] [Google Scholar]
- Rosen C. A., Sodroski J. G., Haseltine W. A. The location of cis-acting regulatory sequences in the human T cell lymphotropic virus type III (HTLV-III/LAV) long terminal repeat. Cell. 1985 Jul;41(3):813–823. doi: 10.1016/s0092-8674(85)80062-3. [DOI] [PubMed] [Google Scholar]
- Shearer G. M., Bernstein D. C., Tung K. S., Via C. S., Redfield R., Salahuddin S. Z., Gallo R. C. A model for the selective loss of major histocompatibility complex self-restricted T cell immune responses during the development of acquired immune deficiency syndrome (AIDS). J Immunol. 1986 Oct 15;137(8):2514–2521. [PubMed] [Google Scholar]
- Spira T. J., Bozeman L. H., Holman R. C., Warfield D. T., Phillips S. K., Feorino P. M. Micromethod for assaying reverse transcriptase of human T-cell lymphotropic virus type III/lymphadenopathy-associated virus. J Clin Microbiol. 1987 Jan;25(1):97–99. doi: 10.1128/jcm.25.1.97-99.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong-Starkesen S. E., Luciw P. A., Peterlin B. M. Signaling through T lymphocyte surface proteins, TCR/CD3 and CD28, activates the HIV-1 long terminal repeat. J Immunol. 1989 Jan 15;142(2):702–707. [PubMed] [Google Scholar]
- Tsubota H., Lord C. I., Watkins D. I., Morimoto C., Letvin N. L. A cytotoxic T lymphocyte inhibits acquired immunodeficiency syndrome virus replication in peripheral blood lymphocytes. J Exp Med. 1989 Apr 1;169(4):1421–1434. doi: 10.1084/jem.169.4.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdin E., Becker N., Bex F., Droogmans L., Burny A. Identification and characterization of an enhancer in the coding region of the genome of human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4874–4878. doi: 10.1073/pnas.87.12.4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Via C. S., Morse H. C., 3rd, Shearer G. M. Altered immunoregulation and autoimmune aspects of HIV infection: relevant murine models. Immunol Today. 1990 Jul;11(7):250–255. doi: 10.1016/0167-5699(90)90099-u. [DOI] [PubMed] [Google Scholar]
- Walker C. M., Levy J. A. A diffusible lymphokine produced by CD8+ T lymphocytes suppresses HIV replication. Immunology. 1989 Apr;66(4):628–630. [PMC free article] [PubMed] [Google Scholar]
- Walker C. M., Moody D. J., Stites D. P., Levy J. A. CD8+ lymphocytes can control HIV infection in vitro by suppressing virus replication. Science. 1986 Dec 19;234(4783):1563–1566. doi: 10.1126/science.2431484. [DOI] [PubMed] [Google Scholar]