Abstract
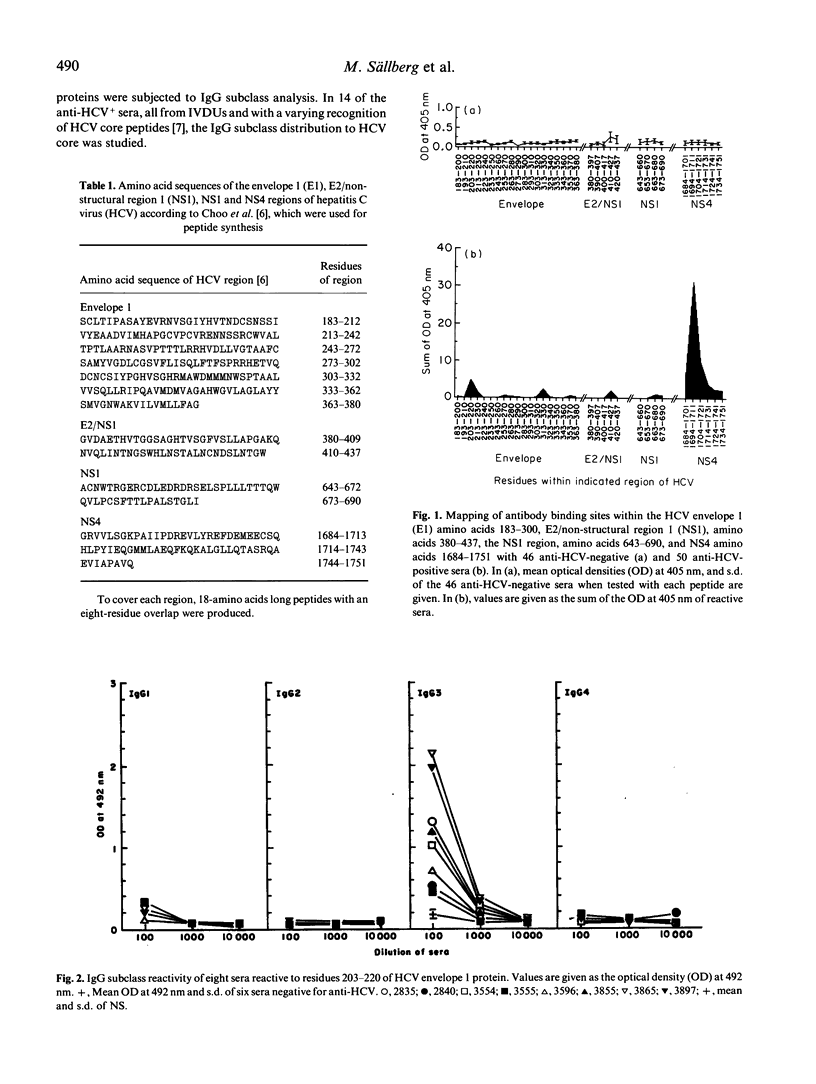
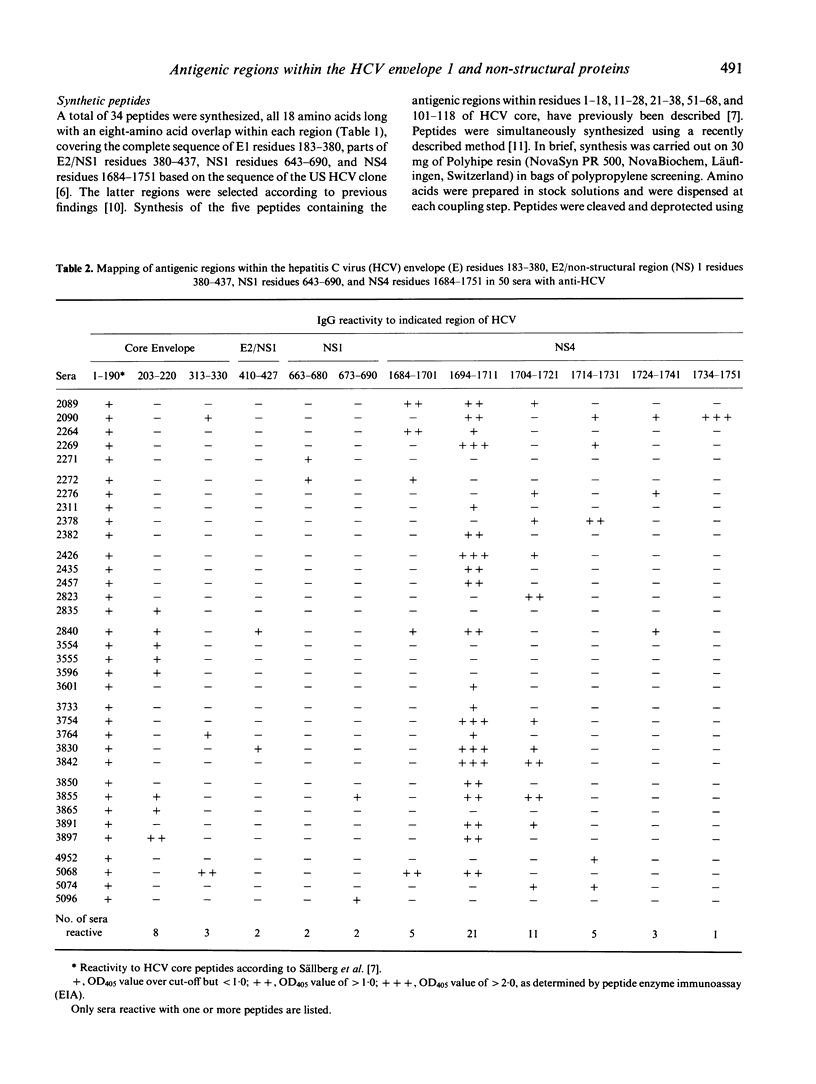
Antibody binding to antigenic regions of hepatitis C virus (HCV) envelope 1 (E1; residues 183-380, E2/non-structural (NS) 1 (residues 380-437), NS1 (residues 643-690), and NS4 (1684-1751) proteins were assayed for 50 sera with antibodies to HCV (anti-HCV) and for 46 sera without anti-HCV. Thirty-four peptides, 18 residues long with an eight-amino acid overlap within each HCV region, were synthesized and tested with all 96 sera. Within the E region 183-380, the major binding site was located to residues 203-220, and was recognized by eight sera. Within the E2/NS1 region 380-437, the peptide covering residues 410-427 was recognized by two sera, and within the NS1 region 643-690, peptides covering residues 663-690 were recognized by four sera. Within the NS4 region 1684-1751, 27 sera were reactive to one or more of the NS4 peptides, and 21 out of these were reactive with peptide 1694-1711. One part of the major binding site could be located to residues 1701-1704, with the sequence Leu-Tyr-Arg-Glu. The IgG1, IgG3 and IgG4 subclasses were reactive with the five antigenic regions of HCV core, residues 1-18, 11-28, 21-38, 51-68 and 101-118. Reactivity to the major envelope site consisted almost exclusively of IgG3, and reactivity to the major site of NS4 consisted only of IgG1. Thus, a non-restricted IgG response to linear HCV-encoded binding sites was found to the core protein, whereas IgG subclass-restricted linear binding sites were found within the E1 protein, and within the NS4 protein.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert J., Franzén L., Jansson M., Scarlatti G., Kataaha P. K., Katabira E., Mubiro F., Rydåker M., Rossi P., Pettersson U. Ugandan HIV-1 V3 loop sequences closely related to the U.S./European consensus. Virology. 1992 Oct;190(2):674–681. doi: 10.1016/0042-6822(92)90905-5. [DOI] [PubMed] [Google Scholar]
- Cerino A., Mondelli M. U. Identification of an immunodominant B cell epitope on the hepatitis C virus nonstructural region defined by human monoclonal antibodies. J Immunol. 1991 Oct 15;147(8):2692–2696. [PubMed] [Google Scholar]
- Chaudhary R. K., MacLean C. Evaluation of first- and second-generation RIBA kits for detection of antibody to hepatitis C virus. J Clin Microbiol. 1991 Oct;29(10):2329–2330. doi: 10.1128/jcm.29.10.2329-2330.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba J., Ohba H., Matsuura Y., Watanabe Y., Katayama T., Kikuchi S., Saito I., Miyamura T. Serodiagnosis of hepatitis C virus (HCV) infection with an HCV core protein molecularly expressed by a recombinant baculovirus. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4641–4645. doi: 10.1073/pnas.88.11.4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching W. M., Wychowski C., Beach M. J., Wang H., Davies C. L., Carl M., Bradley D. W., Alter H. J., Feinstone S. M., Shih J. W. Interaction of immune sera with synthetic peptides corresponding to the structural protein region of hepatitis C virus. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3190–3194. doi: 10.1073/pnas.89.8.3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo Q. L., Richman K. H., Han J. H., Berger K., Lee C., Dong C., Gallegos C., Coit D., Medina-Selby R., Barr P. J. Genetic organization and diversity of the hepatitis C virus. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2451–2455. doi: 10.1073/pnas.88.6.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farci P., Alter H. J., Govindarajan S., Wong D. C., Engle R., Lesniewski R. R., Mushahwar I. K., Desai S. M., Miller R. H., Ogata N. Lack of protective immunity against reinfection with hepatitis C virus. Science. 1992 Oct 2;258(5079):135–140. doi: 10.1126/science.1279801. [DOI] [PubMed] [Google Scholar]
- Kuo G., Choo Q. L., Alter H. J., Gitnick G. L., Redeker A. G., Purcell R. H., Miyamura T., Dienstag J. L., Alter M. J., Stevens C. E. An assay for circulating antibodies to a major etiologic virus of human non-A, non-B hepatitis. Science. 1989 Apr 21;244(4902):362–364. doi: 10.1126/science.2496467. [DOI] [PubMed] [Google Scholar]
- Matsuura Y., Harada S., Suzuki R., Watanabe Y., Inoue Y., Saito I., Miyamura T. Expression of processed envelope protein of hepatitis C virus in mammalian and insect cells. J Virol. 1992 Mar;66(3):1425–1431. doi: 10.1128/jvi.66.3.1425-1431.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson L., Gutierrez R. A., Dawson G. J., Lesniewski R. R., Mushahwar L. K., Weiland O. Antibodies to recombinant and synthetic peptides derived from the hepatitis C virus genome in long-term-studied patients with posttransfusion hepatitis C. Scand J Gastroenterol. 1991 Dec;26(12):1257–1262. doi: 10.3109/00365529108998622. [DOI] [PubMed] [Google Scholar]
- Muraiso K., Hijikata M., Kato N., Shimotohno K., Okazaki N., Ohkoshi S., Uura M., Kaneko S., Kobayashi K., Omata M. Detection of hepatitis C virus infection by enzyme-linked immunosorbent assay system using core protein expressed in Escherichia coli. Jpn J Cancer Res. 1991 Aug;82(8):879–882. doi: 10.1111/j.1349-7006.1991.tb01914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto H., Tsuda F., Machida A., Munekata E., Akahane Y., Sugai Y., Mashiko K., Mitsui T., Tanaka T., Miyakawa Y. Antibodies against synthetic oligopeptides deduced from the putative core gene for the diagnosis of hepatitis virus infection. Hepatology. 1992 Feb;15(2):180–186. doi: 10.1002/hep.1840150203. [DOI] [PubMed] [Google Scholar]
- Skvaril F., Joller-Jemelka H. IgG subclasses of anti-HBs antibodies in vaccinated and nonvaccinated individuals and in anti-HBs immunoglobulin preparations. Int Arch Allergy Appl Immunol. 1984;73(4):330–337. doi: 10.1159/000233493. [DOI] [PubMed] [Google Scholar]
- Sälberg M., Norder H., Weiland O., Magnius L. Immunoglobulin isotypes of anti-HBc and anti-HBe and hepatitis B virus (HBV) DNA elimination in acute hepatitis B. J Med Virol. 1989 Dec;29(4):296–302. doi: 10.1002/jmv.1890290414. [DOI] [PubMed] [Google Scholar]
- Sällberg M., Norder H., Magnius L. O. Comparison of class and subclass distribution of antibodies to the hepatitis B core and B e antigens in chronic hepatitis B. J Med Virol. 1990 Jan;30(1):1–6. doi: 10.1002/jmv.1890300102. [DOI] [PubMed] [Google Scholar]
- Sällberg M., Rudén U., Magnius L. O., Norrby E., Wahren B. Rapid "tea-bag" peptide synthesis using 9-fluorenylmethoxycarbonyl (Fmoc) protected amino acids applied for antigenic mapping of viral proteins. Immunol Lett. 1991 Sep;30(1):59–68. doi: 10.1016/0165-2478(91)90090-w. [DOI] [PubMed] [Google Scholar]
- Sällberg M., Rudén U., Wahren B., Magnius L. O. Immune response to a single peptide containing an immunodominant region of hepatitis C virus core protein: the isotypes and the recognition site. Immunol Lett. 1992 Jun;33(1):27–33. doi: 10.1016/0165-2478(92)90089-7. [DOI] [PubMed] [Google Scholar]
- Sällberg M., Rudén U., Wahren B., Magnius L. O. Immunodominant regions within the hepatitis C virus core and putative matrix proteins. J Clin Microbiol. 1992 Aug;30(8):1989–1994. doi: 10.1128/jcm.30.8.1989-1994.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sällberg M., Rudén U., Wahren B., Noah M., Magnius L. O. Human and murine B-cells recognize the HBeAg/beta (or HBe2) epitope as a linear determinant. Mol Immunol. 1991 Jul;28(7):719–726. doi: 10.1016/0161-5890(91)90114-y. [DOI] [PubMed] [Google Scholar]
- Van der Poel C. L., Cuypers H. T., Reesink H. W., Weiner A. J., Quan S., Di Nello R., Van Boven J. J., Winkel I., Mulder-Folkerts D., Exel-Oehlers P. J. Confirmation of hepatitis C virus infection by new four-antigen recombinant immunoblot assay. Lancet. 1991 Feb 9;337(8737):317–319. doi: 10.1016/0140-6736(91)90942-i. [DOI] [PubMed] [Google Scholar]
- Weiner A. J., Brauer M. J., Rosenblatt J., Richman K. H., Tung J., Crawford K., Bonino F., Saracco G., Choo Q. L., Houghton M. Variable and hypervariable domains are found in the regions of HCV corresponding to the flavivirus envelope and NS1 proteins and the pestivirus envelope glycoproteins. Virology. 1991 Feb;180(2):842–848. doi: 10.1016/0042-6822(91)90104-j. [DOI] [PubMed] [Google Scholar]
- Weiner A. J., Geysen H. M., Christopherson C., Hall J. E., Mason T. J., Saracco G., Bonino F., Crawford K., Marion C. D., Crawford K. A. Evidence for immune selection of hepatitis C virus (HCV) putative envelope glycoprotein variants: potential role in chronic HCV infections. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3468–3472. doi: 10.1073/pnas.89.8.3468. [DOI] [PMC free article] [PubMed] [Google Scholar]



