Abstract
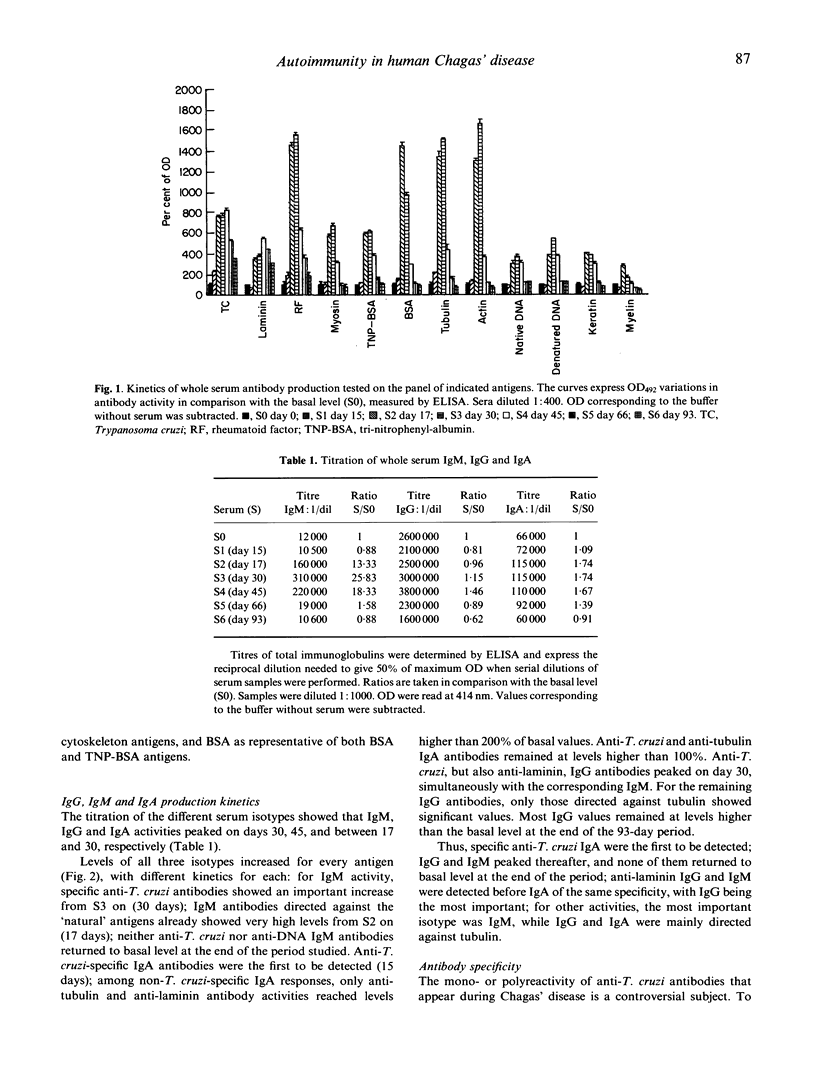
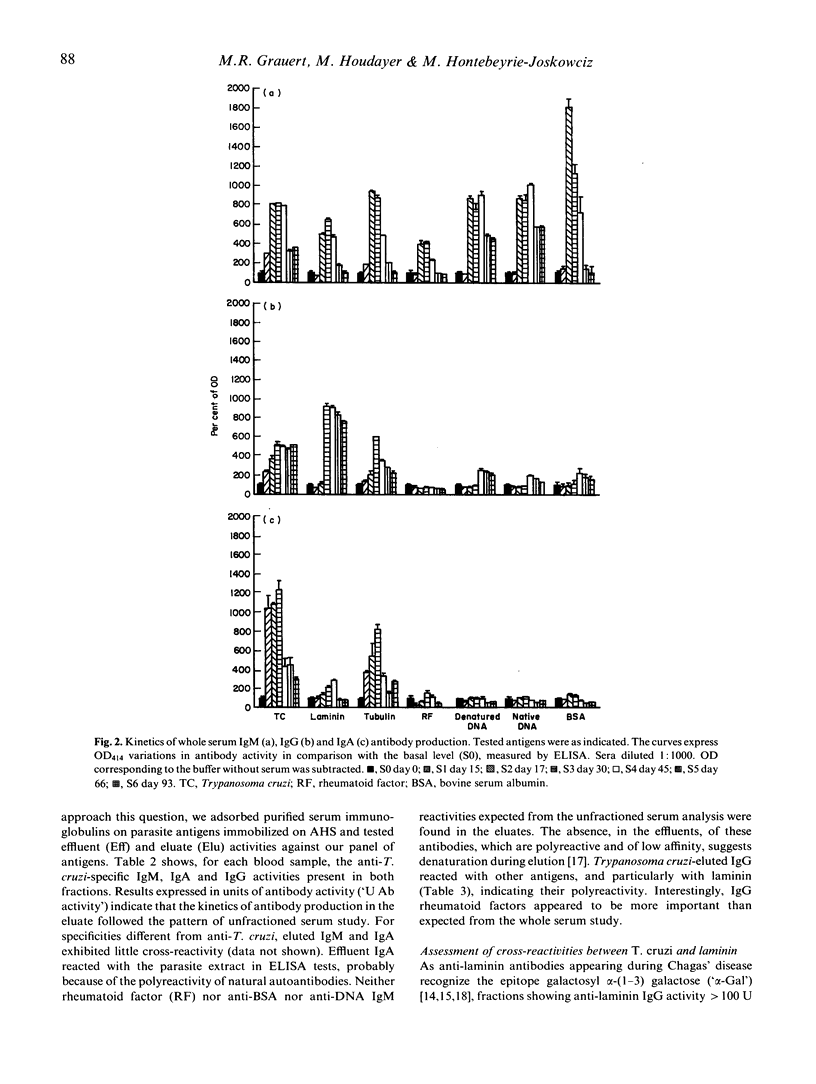
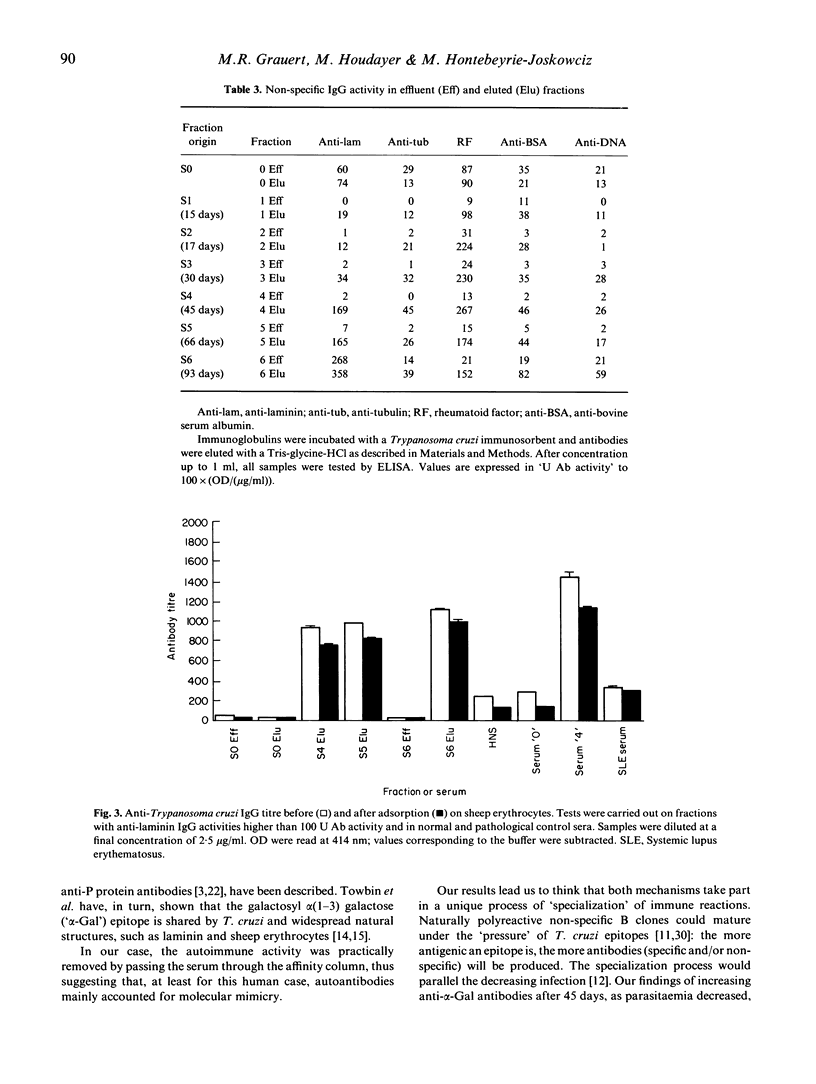
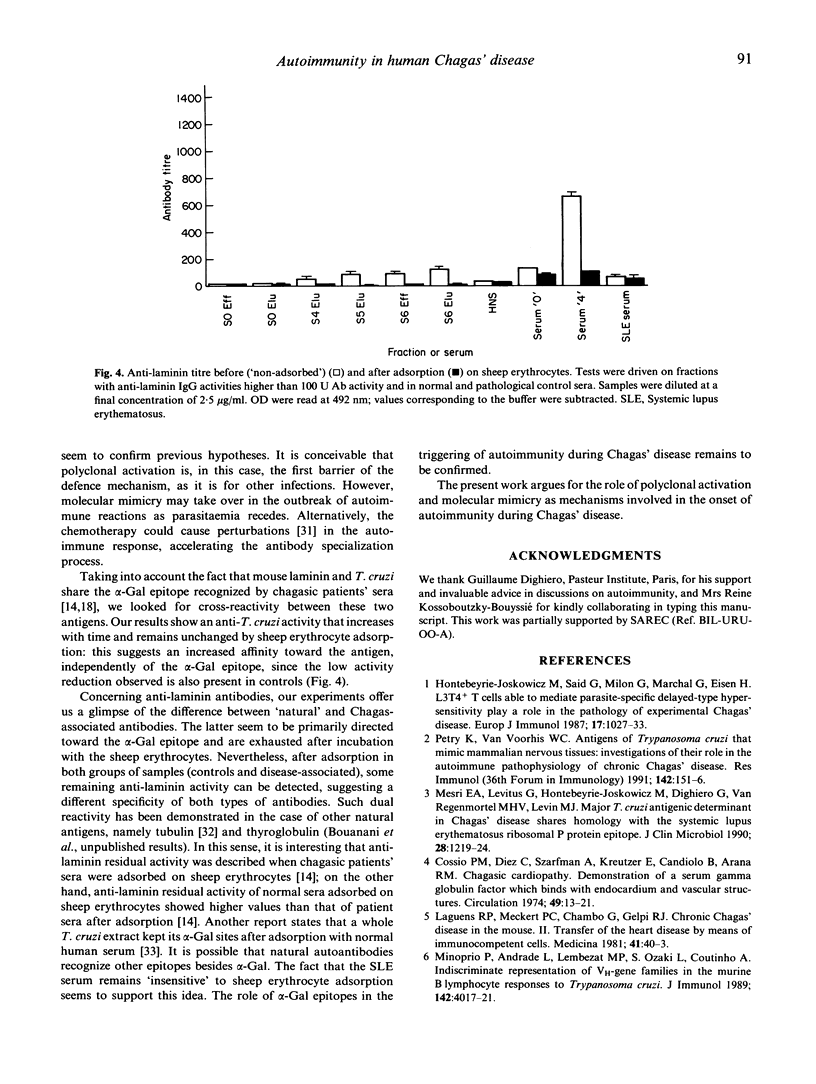
The kinetics of antibody response in an acute case of human Chagas' disease was investigated. Hypergammaglubulinaemia appeared at day 17 of infection, and persisted after 66 days of infection, at which time parasitaemia became undetectable. Titration of immunoglobulins showed that the three principal isotypes were involved in the response, emphasizing polyclonal B cell activation. Total IgA was detected before total IgM, and the latter before total IgG. High titres of autoantibodies were found among IgM and IgG subclasses. IgA was also the first isotype to be detected among specific anti-Trypanosoma cruzi antibodies. However, the maximal parasite antibody response was attained after 30 days of infection for all isotypes. With regard to possible cross-reactivity between molecules of host and parasite, adsorption experiments on T. cruzi-specific immunosorbent were designed. Specific antibodies, present in the eluates, also recognized natural antigens, especially laminin. In order to characterize the alpha-galactose epitope of laminin, adsorption experiments on sheep erythrocytes were performed, and revealed the possible presence of another epitope on the glycoprotein. Our results indicate that in the case of Chagas' disease investigated here, polyclonal activation occurred; moreover, they suggest that molecular mimicry may play a role by increasing autoantibodies, probably via a parasite-driven mechanism.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adib M., Ragimbeau J., Avrameas S., Ternynck T. IgG autoantibody activity in normal mouse serum is controlled by IgM. J Immunol. 1990 Dec 1;145(11):3807–3813. [PubMed] [Google Scholar]
- Almeida I. C., Milani S. R., Gorin P. A., Travassos L. R. Complement-mediated lysis of Trypanosoma cruzi trypomastigotes by human anti-alpha-galactosyl antibodies. J Immunol. 1991 Apr 1;146(7):2394–2400. [PubMed] [Google Scholar]
- Avila J. L., Rojas M., Towbin H. Serological activity against galactosyl-alpha(1-3)galactose in sera from patients with several kinetoplastida infections. J Clin Microbiol. 1988 Jan;26(1):126–132. doi: 10.1128/jcm.26.1.126-132.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossio P. M., Diez C., Szarfman A., Kreutzer E., Candiolo B., Arana R. M. Chagasic cardiopathy. Demonstration of a serum gamma globulin factor which reacts with endocardium and vascular structures. Circulation. 1974 Jan;49(1):13–21. doi: 10.1161/01.cir.49.1.13. [DOI] [PubMed] [Google Scholar]
- Cunningham D. S., Kuhn R. E. Lymphoblast transformation as a measure of immune competence during experimental Chagas' disease. J Parasitol. 1980 Jun;66(3):390–398. [PubMed] [Google Scholar]
- Dziarski R. Autoimmunity: polyclonal activation or antigen induction? Immunol Today. 1988 Nov;9(11):340–342. doi: 10.1016/0167-5699(88)91333-3. [DOI] [PubMed] [Google Scholar]
- Gazzinelli R. T. Natural anti-Gal antibodies prevent, rather than cause, autoimmunity in human Chagas' disease. Res Immunol. 1991 Feb;142(2):164–167. doi: 10.1016/0923-2494(91)90031-d. [DOI] [PubMed] [Google Scholar]
- Hanson W. L., Devlin R. F., Roberson E. L. Immunoglobulin levels in a laboratory-acquired case of human Chagas' disease. J Parasitol. 1974 Jun;60(3):532–533. [PubMed] [Google Scholar]
- Hontebeyrie-Joskowicz M., Said G., Milon G., Marchal G., Eisen H. L3T4+ T cells able to mediate parasite-specific delayed-type hypersensitivity play a role in the pathology of experimental Chagas' disease. Eur J Immunol. 1987 Jul;17(7):1027–1033. doi: 10.1002/eji.1830170720. [DOI] [PubMed] [Google Scholar]
- Israelski D. M., Sadler R., Araujo F. G. Antibody response and antigen recognition in human infection with Trypanosoma cruzi. Am J Trop Med Hyg. 1988 Nov;39(5):445–455. doi: 10.4269/ajtmh.1988.39.445. [DOI] [PubMed] [Google Scholar]
- Jerne N. K. THE NATURAL-SELECTION THEORY OF ANTIBODY FORMATION. Proc Natl Acad Sci U S A. 1955 Nov 15;41(11):849–857. doi: 10.1073/pnas.41.11.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerner N., Liégeard P., Levin M. J., Hontebeyrie-Joskowicz M. Trypanosoma cruzi: antibodies to a MAP-like protein in chronic Chagas' disease cross-react with mammalian cytoskeleton. Exp Parasitol. 1991 Nov;73(4):451–459. doi: 10.1016/0014-4894(91)90069-9. [DOI] [PubMed] [Google Scholar]
- Laguens R. P., Meckert P. C., Chambo G., Gelpi R. J. Chronic Chagas disease in the mouse. II. Transfer of the heart disease by means of immunocompetent cells. Medicina (B Aires) 1981;41(1):40–43. [PubMed] [Google Scholar]
- Levin M. J. Molecular mimicry and Chagas' heart disease: high anti-R-13 autoantibody levels are markers of severe Chagas heart complaint. Res Immunol. 1991 Feb;142(2):157–159. doi: 10.1016/0923-2494(91)90029-i. [DOI] [PubMed] [Google Scholar]
- Little J. R., Eisen H. N. Preparation and characterization of antibodies specific for the 2,4,6-trinitrophenyl group. Biochemistry. 1966 Nov;5(11):3385–3395. doi: 10.1021/bi00875a001. [DOI] [PubMed] [Google Scholar]
- Matthes T., Wolff A., Soubiran P., Gros F., Dighiero G. Antitubulin antibodies. II. Natural autoantibodies and induced antibodies recognize different epitopes on the tubulin molecule. J Immunol. 1988 Nov 1;141(9):3135–3141. [PubMed] [Google Scholar]
- Mesri E. A., Levitus G., Hontebeyrie-Joskowicz M., Dighiero G., Van Regenmortel M. H., Levin M. J. Major Trypanosoma cruzi antigenic determinant in Chagas' heart disease shares homology with the systemic lupus erythematosus ribosomal P protein epitope. J Clin Microbiol. 1990 Jun;28(6):1219–1224. doi: 10.1128/jcm.28.6.1219-1224.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minoprio P., Andrade L., Lembezat M. P., Ozaki L. S., Coutinho A. Indiscriminate representation of VH-gene families in the murine B lymphocyte responses to Trypanosoma cruzi. J Immunol. 1989 Jun 1;142(11):4017–4021. [PubMed] [Google Scholar]
- Ortiz-Ortiz L., Parks D. E., Rodriguez M., Weigle W. O. Polyclonal B lymphocyte activation during Trypanosoma cruzi infection. J Immunol. 1980 Jan;124(1):121–126. [PubMed] [Google Scholar]
- Petry K., Van Voorhis W. C. Antigens of Trypanosoma cruzi that mimic mammalian nervous tissues: investigations of their role in the autoimmune pathophysiology of chronic Chagas' disease. Res Immunol. 1991 Feb;142(2):151–156. doi: 10.1016/0923-2494(91)90028-h. [DOI] [PubMed] [Google Scholar]
- Primavera K. S., Umezawa E. S., Peres B. A., Camargo M. E., Hoshino-Shimizu S. Chagas'disease: IgA, IgM and IgG antibodies to T. cruzi amastigote, trypomastigote and epimastigote antigens in acute and in different chronic forms of the disease. Rev Inst Med Trop Sao Paulo. 1990 May-Jun;32(3):172–180. doi: 10.1590/s0036-46651990000300005. [DOI] [PubMed] [Google Scholar]
- Shlomchik M. J., Marshak-Rothstein A., Wolfowicz C. B., Rothstein T. L., Weigert M. G. The role of clonal selection and somatic mutation in autoimmunity. 1987 Aug 27-Sep 2Nature. 328(6133):805–811. doi: 10.1038/328805a0. [DOI] [PubMed] [Google Scholar]
- Spinella S., Liegeard P., Hontebeyrie-Joskowicz M. Trypanosoma cruzi: predominance of IgG2a in nonspecific humoral response during experimental Chagas' disease. Exp Parasitol. 1992 Feb;74(1):46–56. doi: 10.1016/0014-4894(92)90138-z. [DOI] [PubMed] [Google Scholar]
- Tarleton R. L., Kuhn R. E. Measurement of parasite-specific immune responses in vitro: evidence for suppression of the antibody response to Trypanosoma cruzi. Eur J Immunol. 1985 Aug;15(8):845–850. doi: 10.1002/eji.1830150820. [DOI] [PubMed] [Google Scholar]
- Ternynck T., Bleux C., Gregoire J., Avrameas S., Kanellopoulos-Langevin C. Comparison between autoantibodies arising during Trypanosoma cruzi infection in mice and natural autoantibodies. J Immunol. 1990 Feb 15;144(4):1504–1511. [PubMed] [Google Scholar]
- Torrico F., Heremans H., Rivera M. T., Van Marck E., Billiau A., Carlier Y. Endogenous IFN-gamma is required for resistance to acute Trypanosoma cruzi infection in mice. J Immunol. 1991 May 15;146(10):3626–3632. [PubMed] [Google Scholar]
- Towbin H., Rosenfelder G., Wieslander J., Avila J. L., Rojas M., Szarfman A., Esser K., Nowack H., Timpl R. Circulating antibodies to mouse laminin in Chagas disease, American cutaneous leishmaniasis, and normal individuals recognize terminal galactosyl(alpha 1-3)-galactose epitopes. J Exp Med. 1987 Aug 1;166(2):419–432. doi: 10.1084/jem.166.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Imperio Lima M. R., Eisen H., Minoprio P., Joskowicz M., Coutinho A. Persistence of polyclonal B cell activation with undetectable parasitemia in late stages of experimental Chagas' disease. J Immunol. 1986 Jul 1;137(1):353–356. [PubMed] [Google Scholar]


