Abstract
The accumulation of inflammatory cells in synovial tissue was studied using indirect immunofluorescence assays on cell cultures and frozen tissue sections of healing rat digital flexor tendons. Flexor tendons were collected from rats 3, 7 and 14 days after crush injury. Tendon sheath and epithenon cells were isolated by sequential enzymic digestion and cultured for 2 days. Subpopulations of synovial and inflammatory cells were identified with MoAbs against cell surface glycoproteins present on B lymphocytes (CD45), T lymphocytes (CD2, CD4, CD8), macrophages (CD14) and endothelial cells. A phagocytosis assay was also used to identify macrophages. We report a substantial increase in the number of T lymphocytes (mainly helper/inducer) and phagocytotic cells with monocyte/macrophage surface markers in tendon sheath and epitenon 3 days after crush injury. The infiltration of inflammatory cells into synovial sheath and epitenon preceded an increase in fibronectin production by tendon cells which was seen 7 days after injury. To study the interaction between T lymphocytes and synovial cells in vitro, we established synovial fibroblast-like type B cell cultures and used stimulated and non-stimulated T lymphocytes in cell binding assays. We observed increased adhesiveness between unstimulated synovial cells and synovial cells previously cultured with activated and non-activated T lymphocytes. ELISA inhibition studies have shown an increase in fibronectin production by synovial fibroblasts co-cultured with stimulated CD4+ T lymphocytes. We suggest that the presence of inflammatory cells in synovial sheath and epitenon during tendon healing induces synovial fibroblasts and epitenon cells to increase their production of fibronectin, which provides a scaffold for subsequent adhesion formation.
Full text
PDF

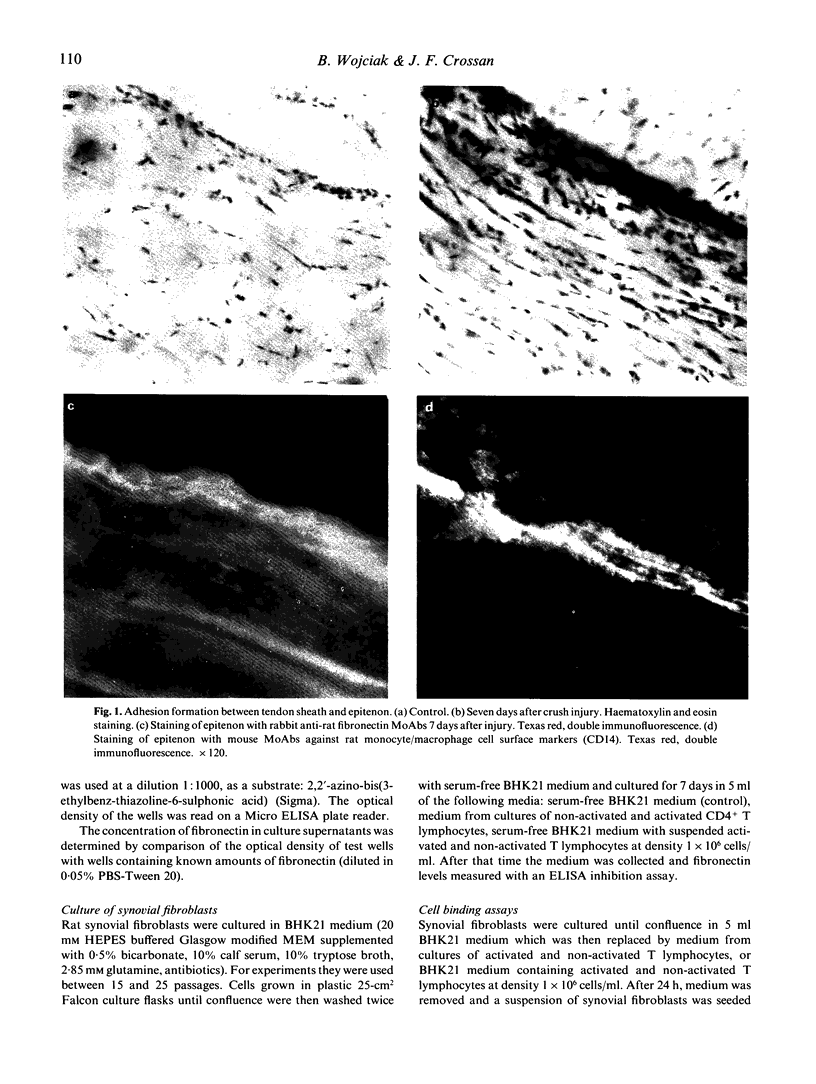
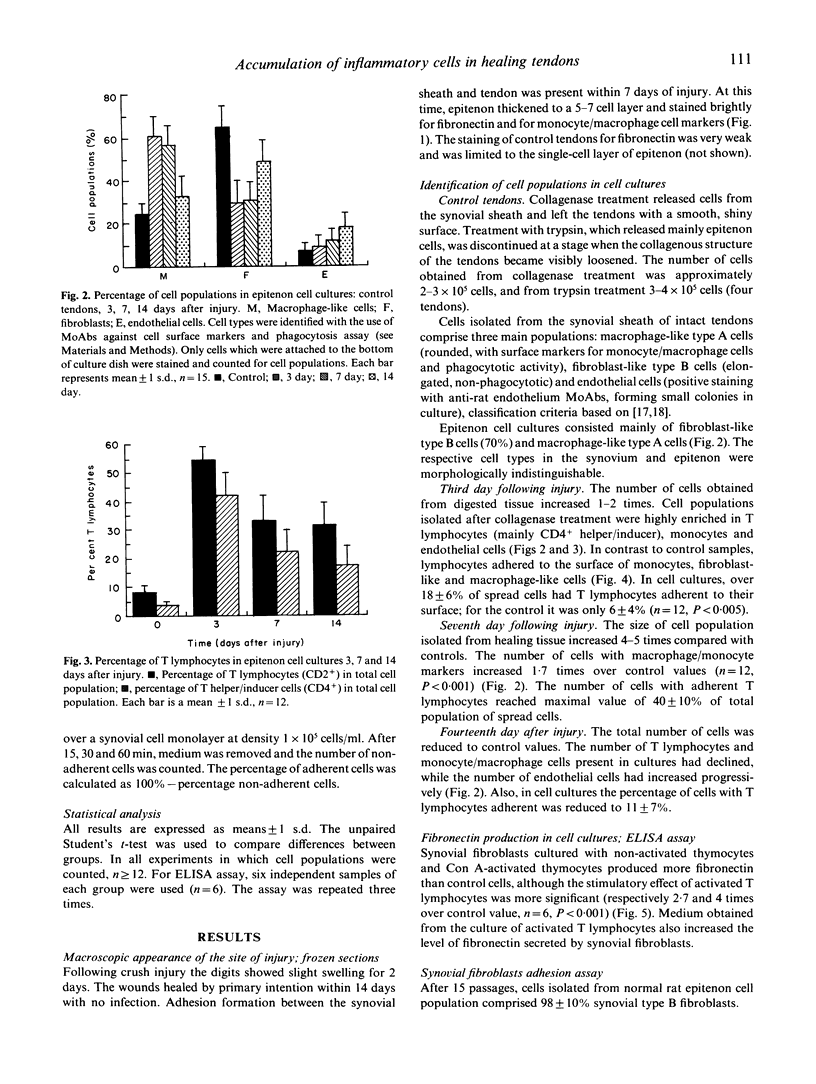
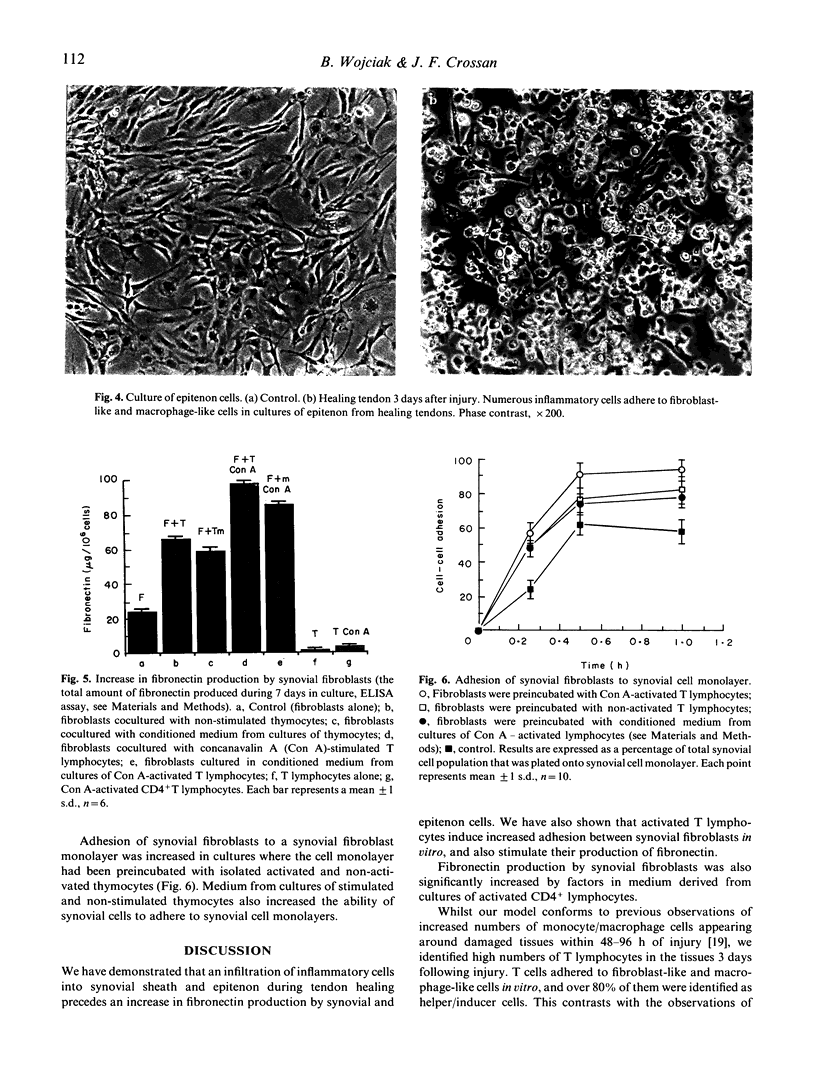


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaron J. E., Carter D. H. Rapid preparation of fresh-frozen undecalcified bone for histological and histochemical analysis. J Histochem Cytochem. 1987 Mar;35(3):361–369. doi: 10.1177/35.3.2434557. [DOI] [PubMed] [Google Scholar]
- Agelli M., Wahl S. M. Cytokines and fibrosis. Clin Exp Rheumatol. 1986 Oct-Dec;4(4):379–388. [PubMed] [Google Scholar]
- Babu M., Diegelmann R., Oliver N. Fibronectin is overproduced by keloid fibroblasts during abnormal wound healing. Mol Cell Biol. 1989 Apr;9(4):1642–1650. doi: 10.1128/mcb.9.4.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banes A. J., Donlon K., Link G. W., Gillespie Y., Bevin A. G., Peterson H. D., Bynum D., Watts S., Dahners L. Cell populations of tendon: a simplified method for isolation of synovial cells and internal fibroblasts: confirmation of origin and biologic properties. J Orthop Res. 1988;6(1):83–94. doi: 10.1002/jor.1100060111. [DOI] [PubMed] [Google Scholar]
- Banes A. J., Link G. W., Bevin A. G., Peterson H. D., Gillespie Y., Bynum D., Watts S., Dahners L. Tendon synovial cells secrete fibronectin in vivo and in vitro. J Orthop Res. 1988;6(1):73–82. doi: 10.1002/jor.1100060110. [DOI] [PubMed] [Google Scholar]
- Barbul A., Breslin R. J., Woodyard J. P., Wasserkrug H. L., Efron G. The effect of in vivo T helper and T suppressor lymphocyte depletion on wound healing. Ann Surg. 1989 Apr;209(4):479–483. doi: 10.1097/00000658-198904000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbul A., Knud-Hansen J., Wasserkrug H. L., Efron G. Interleukin 2 enhances wound healing in rats. J Surg Res. 1986 Apr;40(4):315–319. doi: 10.1016/0022-4804(86)90193-9. [DOI] [PubMed] [Google Scholar]
- Becker H., Langrock A., Federlin K. Imbalance of CD4+ lymphocyte subsets in patients with mixed connective tissue disease. Clin Exp Immunol. 1992 Apr;88(1):91–95. doi: 10.1111/j.1365-2249.1992.tb03044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. A., Quinn J. H., Winn H. J., Lanigan J. M., Dellepella P., Colvin R. B. Fibronectin is produced by blood vessels in response to injury. J Exp Med. 1982 Aug 1;156(2):646–651. doi: 10.1084/jem.156.2.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards J. C. The origin of type A synovial lining cells. Immunobiology. 1982 Apr;161(3-4):227–231. doi: 10.1016/S0171-2985(82)80078-8. [DOI] [PubMed] [Google Scholar]
- Gelberman R. H., Steinberg D., Amiel D., Akeson W. Fibroblast chemotaxis after tendon repair. J Hand Surg Am. 1991 Jul;16(4):686–693. doi: 10.1016/0363-5023(91)90195-h. [DOI] [PubMed] [Google Scholar]
- Gelberman R. H., Vande Berg J. S., Lundborg G. N., Akeson W. H. Flexor tendon healing and restoration of the gliding surface. An ultrastructural study in dogs. J Bone Joint Surg Am. 1983 Jan;65(1):70–80. [PubMed] [Google Scholar]
- Gerdes J. S., Douglas S. D., Kolski G. B., Yoder M. C., Polin R. A. Decreased fibronectin biosynthesis by human cord blood mononuclear phagocytes in vitro. J Leukoc Biol. 1984 Jan;35(1):91–99. doi: 10.1002/jlb.35.1.91. [DOI] [PubMed] [Google Scholar]
- Haynes B. F., Grover B. J., Whichard L. P., Hale L. P., Nunley J. A., McCollum D. E., Singer K. H. Synovial microenvironment-T cell interactions. Human T cells bind to fibroblast-like synovial cells in vitro. Arthritis Rheum. 1988 Aug;31(8):947–955. doi: 10.1002/art.1780310802. [DOI] [PubMed] [Google Scholar]
- Kang H. J., Park B. M., Hahn S. B., Kang E. S. An experimental study of healing of the partially severed flexor tendon in chickens. Yonsei Med J. 1990 Sep;31(3):264–273. doi: 10.3349/ymj.1990.31.3.264. [DOI] [PubMed] [Google Scholar]
- Kehrl J. H., Wakefield L. M., Roberts A. B., Jakowlew S., Alvarez-Mon M., Derynck R., Sporn M. B., Fauci A. S. Production of transforming growth factor beta by human T lymphocytes and its potential role in the regulation of T cell growth. J Exp Med. 1986 May 1;163(5):1037–1050. doi: 10.1084/jem.163.5.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krummel T. M., Michna B. A., Thomas B. L., Sporn M. B., Nelson J. M., Salzberg A. M., Cohen I. K., Diegelmann R. F. Transforming growth factor beta (TGF-beta) induces fibrosis in a fetal wound model. J Pediatr Surg. 1988 Jul;23(7):647–652. doi: 10.1016/s0022-3468(88)80638-9. [DOI] [PubMed] [Google Scholar]
- LINDSAY W. K., BIRCH J. R. THE FIBROBLAST IN FLEXOR TENDON HEALING. Plast Reconstr Surg. 1964 Sep;34:223–232. doi: 10.1097/00006534-196409000-00001. [DOI] [PubMed] [Google Scholar]
- LINDSAY W. K., THOMSON H. G. Digital flexor tendons: an experimental study. Part I. The significance of each component of the flexor mechanismhin tendon healing. Br J Plast Surg. 1960 Jan;12:289–316. doi: 10.1016/s0007-1226(59)80045-x. [DOI] [PubMed] [Google Scholar]
- Lundborg G., Hansson H. A., Rank F., Rydevik B. Superficial repair of severed flexor tendons in synovial environment. An experimental, ultrastructural study on cellular mechanisms. J Hand Surg Am. 1980 Sep;5(5):451–461. doi: 10.1016/s0363-5023(80)80075-x. [DOI] [PubMed] [Google Scholar]
- Martinez-Hernandez A. The hepatic extracellular matrix. II. Electron immunohistochemical studies in rats with CCl4-induced cirrhosis. Lab Invest. 1985 Aug;53(2):166–186. [PubMed] [Google Scholar]
- Mass D. P., Tuel R. J. Participation of human superficialis flexor tendon segments in repair in vitro. J Orthop Res. 1990 Jan;8(1):21–34. doi: 10.1002/jor.1100080104. [DOI] [PubMed] [Google Scholar]
- McDonald J. A., Kelley D. G., Broekelmann T. J. Role of fibronectin in collagen deposition: Fab' to the gelatin-binding domain of fibronectin inhibits both fibronectin and collagen organization in fibroblast extracellular matrix. J Cell Biol. 1982 Feb;92(2):485–492. doi: 10.1083/jcb.92.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POTENZA A. D. Tendon healing within the flexor digital sheath in the dog. J Bone Joint Surg Am. 1962 Jan;44-A:49–64. [PubMed] [Google Scholar]
- Peterson J. M., Barbul A., Breslin R. J., Wasserkrug H. L., Efron G. Significance of T-lymphocytes in wound healing. Surgery. 1987 Aug;102(2):300–305. [PubMed] [Google Scholar]
- Pitzalis C., Kingsley G., Haskard D., Panayi G. The preferential accumulation of helper-inducer T lymphocytes in inflammatory lesions: evidence for regulation by selective endothelial and homotypic adhesion. Eur J Immunol. 1988 Sep;18(9):1397–1404. doi: 10.1002/eji.1830180915. [DOI] [PubMed] [Google Scholar]
- Potenza A. D., Herte M. C. The synovial cavity as a "tissue culture in situ"--science or nonsense? J Hand Surg Am. 1982 Mar;7(2):196–199. doi: 10.1016/s0363-5023(82)80088-9. [DOI] [PubMed] [Google Scholar]
- Raghow R., Lurie S., Seyer J. M., Kang A. H. Profiles of steady state levels of messenger RNAs coding for type I procollagen, elastin, and fibronectin in hamster lungs undergoing bleomycin-induced interstitial pulmonary fibrosis. J Clin Invest. 1985 Nov;76(5):1733–1739. doi: 10.1172/JCI112163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothkopf D. M., Webb S., Szabo R. M., Gelberman R. H., May J. W., Jr An experimental model for the study of canine flexor tendon adhesions. J Hand Surg Am. 1991 Jul;16(4):694–700. doi: 10.1016/0363-5023(91)90196-i. [DOI] [PubMed] [Google Scholar]
- Singer I. I., Kawka D. W., Kazazis D. M., Clark R. A. In vivo co-distribution of fibronectin and actin fibers in granulation tissue: immunofluorescence and electron microscope studies of the fibronexus at the myofibroblast surface. J Cell Biol. 1984 Jun;98(6):2091–2106. doi: 10.1083/jcb.98.6.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl S. M. Host immune factors regulating fibrosis. Ciba Found Symp. 1985;114:175–195. doi: 10.1002/9780470720950.ch12. [DOI] [PubMed] [Google Scholar]
- van Furth R., Diesselhoff-den Dulk M. M. Method to prove investigation of particles by macrophages with light microscopy. Scand J Immunol. 1980;12(3):265–269. doi: 10.1111/j.1365-3083.1980.tb00066.x. [DOI] [PubMed] [Google Scholar]