Abstract
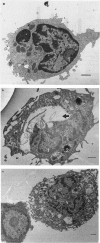
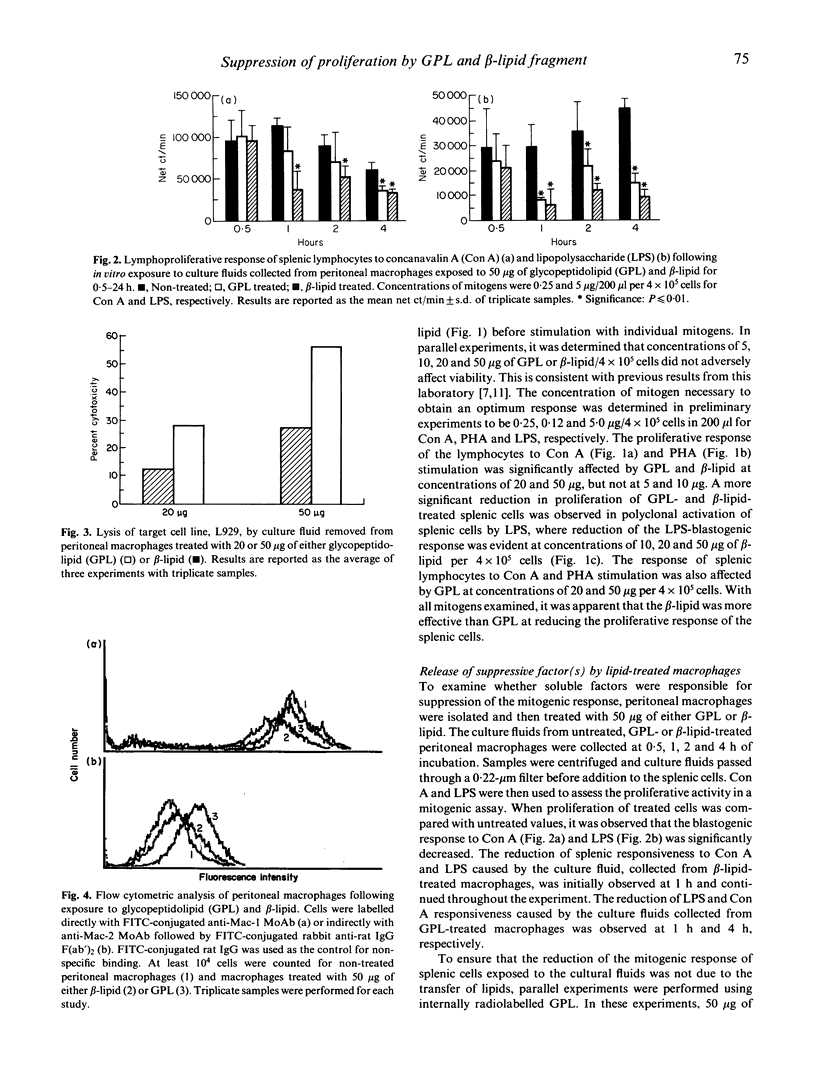
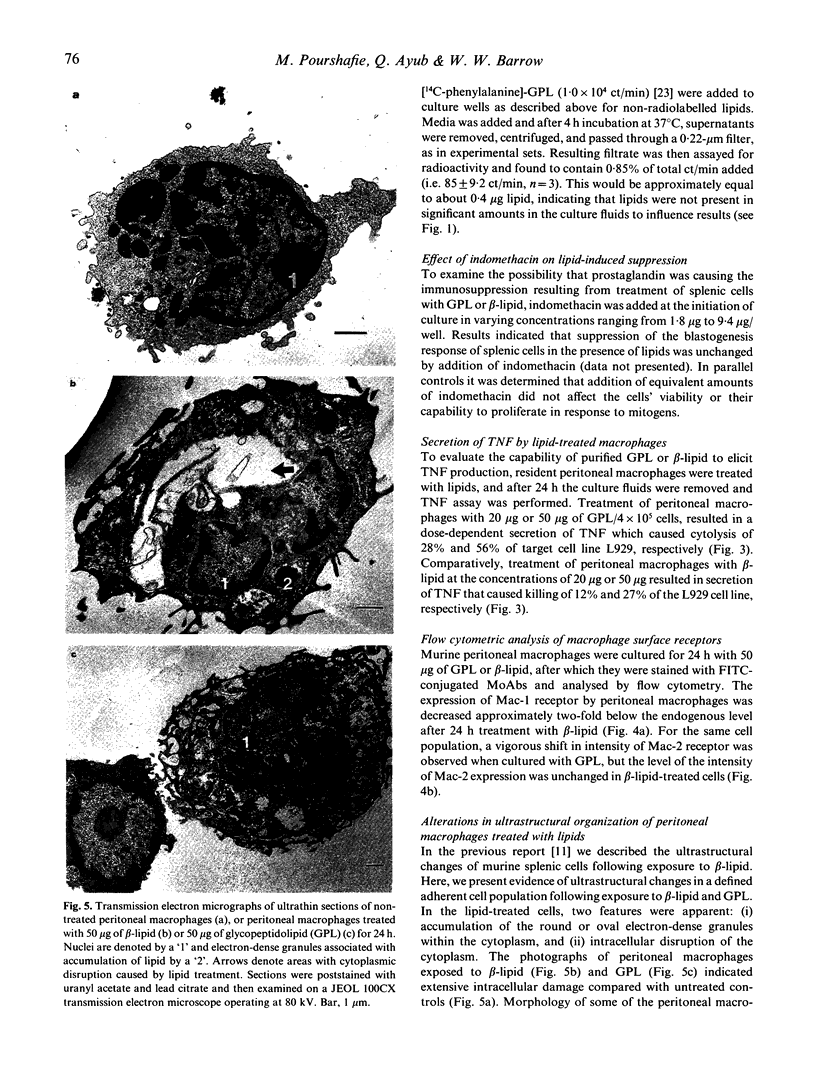
Among the various lipids associated with the cell envelope of the Mycobacterium avium complex, the species-specific glycopeptidolipids (GPL) are responsible for distinguishing one serovar from another. In a continuing effort to study the immunomodulatory capabilities of these mycobacterial lipids, we have examined and compared the effects of the GPL and its lipopeptide fragment (beta-lipid) on mononuclear cell function. It was observed that the lymphoproliferative response of murine splenic mononuclear cells to mitogen stimulation was reduced by both the GPL and its lipopeptide fragment. Although the responsiveness appeared to be down-regulated to a greater degree by the beta-lipid, treatment with either GPL or beta-lipid resulted in the release of soluble factors from peritoneal macrophages that caused suppression of the lymphoproliferative responsiveness of splenic mononuclear cells. Flow cytometric analysis of peritoneal macrophages revealed that treatment with the beta-lipid fragment caused a marked decrease in expression of the C3bi complement receptor, Mac-1, on macrophages, whereas treatment with GPL resulted in a marked increase in the expression of Mac-2 receptor on macrophages. Treatment of peritoneal macrophages with either GPL or beta-lipid resulted in the release of tumour necrosis factor (TNF), as determined by an L929 biological cytotoxicity assay. Perturbation of macrophage membrane ultrastructure by both GPL and beta-lipid was confirmed by electron microscopy, and may be a possible explanation for the resulting alterations in mononuclear cell function observed in this study.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrow W. W. Contributing factors of pathogenesis in the Mycobacterium avium complex. Res Microbiol. 1991 May;142(4):427–433. doi: 10.1016/0923-2508(91)90115-q. [DOI] [PubMed] [Google Scholar]
- Barrow W. W., Ullom B. P., Brennan P. J. Peptidoglycolipid nature of the superficial cell wall sheath of smooth-colony-forming mycobacteria. J Bacteriol. 1980 Nov;144(2):814–822. doi: 10.1128/jb.144.2.814-822.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez L. E., Young L. S. Killing of Mycobacterium avium: insights provided by the use of recombinant cytokines. Res Microbiol. 1990 Feb;141(2):241–243. doi: 10.1016/0923-2508(90)90037-q. [DOI] [PubMed] [Google Scholar]
- Brennan P. J., Aspinall G. O., Shin J. E. Structure of the specific oligosaccharides from the glycopeptidolipid antigens of serovars in the Mycobacterium avium-Mycobacterium intracellulare-Mycobacterium scrofulaceum complex. J Biol Chem. 1981 Jul 10;256(13):6817–6822. [PubMed] [Google Scholar]
- Brennan P. J., Goren M. B. Structural studies on the type-specific antigens and lipids of the mycobacterium avium. Mycobacterium intracellulare. Mycobacterium scrofulaceum serocomplex. Mycobacterium intracellulare serotype 9. J Biol Chem. 1979 May 25;254(10):4205–4211. [PubMed] [Google Scholar]
- Brett S. J., Lowe C., Payne S. N., Draper P. Phenolic glycolipid 1 of Mycobacterium leprae causes nonspecific inflammation but has no effect on cell-mediated responses in mice. Infect Immun. 1984 Dec;46(3):802–808. doi: 10.1128/iai.46.3.802-808.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownback P. E., Barrow W. W. Modified lymphocyte response to mitogens after intraperitoneal injection of glycopeptidolipid antigens from Mycobacterium avium complex. Infect Immun. 1988 May;56(5):1044–1050. doi: 10.1128/iai.56.5.1044-1050.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho de Sousa J. P., Bachelet M., Rastogi N. Effect of indomethacin on the modulation of Mycobacterium avium growth in human macrophages by interferon gamma, retinoic acid and 1,25(OH)2-vitamin D3. FEMS Microbiol Immunol. 1992 Jul;4(5):281–286. doi: 10.1111/j.1574-6968.1992.tb05007.x. [DOI] [PubMed] [Google Scholar]
- Dimitrijevich S. D., Johnson M. M., Barrow W. W. One-step column chromatographic procedure for purification of mycobacterial glycopeptidolipid antigens. J Chromatogr. 1986 Apr 25;377:345–349. doi: 10.1016/s0378-4347(00)80791-4. [DOI] [PubMed] [Google Scholar]
- Draper P., Rees R. J. Electron-transparent zone of mycobacteria may be a defence mechanism. Nature. 1970 Nov 28;228(5274):860–861. doi: 10.1038/228860a0. [DOI] [PubMed] [Google Scholar]
- Drevets D. A., Campbell P. A. Roles of complement and complement receptor type 3 in phagocytosis of Listeria monocytogenes by inflammatory mouse peritoneal macrophages. Infect Immun. 1991 Aug;59(8):2645–2652. doi: 10.1128/iai.59.8.2645-2652.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellner J. J. Sources of variability in assays of the interaction of mycobacteria with mononuclear phagocytes: of mice and men. Res Microbiol. 1990 Feb;141(2):237–240. doi: 10.1016/0923-2508(90)90036-p. [DOI] [PubMed] [Google Scholar]
- Filley E. A., Rook G. A. Effect of mycobacteria on sensitivity to the cytotoxic effects of tumor necrosis factor. Infect Immun. 1991 Aug;59(8):2567–2572. doi: 10.1128/iai.59.8.2567-2572.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flick D. A., Gifford G. E. Comparison of in vitro cell cytotoxic assays for tumor necrosis factor. J Immunol Methods. 1984 Mar 30;68(1-2):167–175. doi: 10.1016/0022-1759(84)90147-9. [DOI] [PubMed] [Google Scholar]
- Fournie J. J., Adams E., Mullins R. J., Basten A. Inhibition of human lymphoproliferative responses by mycobacterial phenolic glycolipids. Infect Immun. 1989 Nov;57(11):3653–3659. doi: 10.1128/iai.57.11.3653-3659.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frehel C., de Chastellier C., Lang T., Rastogi N. Evidence for inhibition of fusion of lysosomal and prelysosomal compartments with phagosomes in macrophages infected with pathogenic Mycobacterium avium. Infect Immun. 1986 Apr;52(1):252–262. doi: 10.1128/iai.52.1.252-262.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangadharam P. R., Edwards C. K., 3rd Release of superoxide anion from resident and activated mouse peritoneal macrophages infected with Mycobacterium intracellulare. Am Rev Respir Dis. 1984 Nov;130(5):834–838. doi: 10.1164/arrd.1984.130.5.834. [DOI] [PubMed] [Google Scholar]
- Ho M. K., Springer T. A. Mac-2, a novel 32,000 Mr mouse macrophage subpopulation-specific antigen defined by monoclonal antibodies. J Immunol. 1982 Mar;128(3):1221–1228. [PubMed] [Google Scholar]
- Hogg N. The leukocyte integrins. Immunol Today. 1989 Apr;10(4):111–114. doi: 10.1016/0167-5699(89)90238-7. [DOI] [PubMed] [Google Scholar]
- Hooper L. C., Barrow W. W. Decreased mitogenic response of murine spleen cells following intraperitoneal injection of serovar-specific glycopeptidolipid antigens from the Mycobacterium avium complex. Adv Exp Med Biol. 1988;239:309–325. doi: 10.1007/978-1-4757-5421-6_31. [DOI] [PubMed] [Google Scholar]
- Hooper L. C., Johnson M. M., Khera V. R., Barrow W. W. Macrophage uptake and retention of radiolabeled glycopeptidolipid antigens associated with the superficial L1 layer of Mycobacterium intracellulare serovar 20. Infect Immun. 1986 Oct;54(1):133–141. doi: 10.1128/iai.54.1.133-141.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard R. D., Collins F. M. Immunomodulation of mouse macrophage killing of Mycobacterium avium in vitro. Infect Immun. 1991 Feb;59(2):570–574. doi: 10.1128/iai.59.2.570-574.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughon B. E., Allaudeen H. S., Becker J. M., Current W. L., Feinberg J., Frenkel J. K., Hafner R., Hughes W. T., Laughlin C. A., Meyers J. D. From the National Institutes of Health. Summary of the workshop on future directions in discovery and development of therapeutic agents for opportunistic infections associated with AIDS. J Infect Dis. 1991 Aug;164(2):244–251. doi: 10.1093/infdis/164.2.244. [DOI] [PubMed] [Google Scholar]
- McNeil M., Tsang A. Y., Brennan P. J. Structure and antigenicity of the specific oligosaccharide hapten from the glycopeptidolipid antigen of Mycobacterium avium serotype 4, the dominant Mycobacterium isolated from patients with acquired immune deficiency syndrome. J Biol Chem. 1987 Feb 25;262(6):2630–2635. [PubMed] [Google Scholar]
- Moreno C., Taverne J., Mehlert A., Bate C. A., Brealey R. J., Meager A., Rook G. A., Playfair J. H. Lipoarabinomannan from Mycobacterium tuberculosis induces the production of tumour necrosis factor from human and murine macrophages. Clin Exp Immunol. 1989 May;76(2):240–245. [PMC free article] [PubMed] [Google Scholar]
- Mosser D. M., Springer T. A., Diamond M. S. Leishmania promastigotes require opsonic complement to bind to the human leukocyte integrin Mac-1 (CD11b/CD18). J Cell Biol. 1992 Jan;116(2):511–520. doi: 10.1083/jcb.116.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman G. W., Gan H. X., McCarthy P. L., Jr, Remold H. G. Survival of human macrophages infected with Mycobacterium avium intracellulare correlates with increased production of tumor necrosis factor-alpha and IL-6. J Immunol. 1991 Dec 1;147(11):3942–3948. [PubMed] [Google Scholar]
- Rastogi N., Bachelet M., Carvalho de Sousa J. P. Intracellular growth of Mycobacterium avium in human macrophages is linked to the increased synthesis of prostaglandin E2 and inhibition of the phagosome-lysosome fusions. FEMS Microbiol Immunol. 1992 Jul;4(5):273–279. doi: 10.1111/j.1574-6968.1992.tb05006.x. [DOI] [PubMed] [Google Scholar]
- Rulong S., Aguas A. P., da Silva P. P., Silva M. T. Intramacrophagic Mycobacterium avium bacilli are coated by a multiple lamellar structure: freeze fracture analysis of infected mouse liver. Infect Immun. 1991 Nov;59(11):3895–3902. doi: 10.1128/iai.59.11.3895-3902.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman G., Wadee A. A. Production of a suppressor factor by CD8+ lymphocytes activated by mycobacterial components. Infect Immun. 1991 Aug;59(8):2828–2835. doi: 10.1128/iai.59.8.2828-2835.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sut A., Sirugue S., Sixou S., Lakhdar-Ghazal F., Tocanne J. F., Lanéelle G. Mycobacteria glycolipids as potential pathogenicity effectors: alteration of model and natural membranes. Biochemistry. 1990 Sep 11;29(36):8498–8502. doi: 10.1021/bi00488a042. [DOI] [PubMed] [Google Scholar]
- Tassell S. K., Pourshafie M., Wright E. L., Richmond M. G., Barrow W. W. Modified lymphocyte response to mitogens induced by the lipopeptide fragment derived from Mycobacterium avium serovar-specific glycopeptidolipids. Infect Immun. 1992 Feb;60(2):706–711. doi: 10.1128/iai.60.2.706-711.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tereletsky M. J., Barrow W. W. Postphagocytic detection of glycopeptidolipids associated with the superficial L1 layer of Mycobacterium intracellulare. Infect Immun. 1983 Sep;41(3):1312–1321. doi: 10.1128/iai.41.3.1312-1321.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomioka H., Saito H., Yamada Y. Characteristics of immunosuppressive macrophages induced in spleen cells by Mycobacterium avium complex infections in mice. J Gen Microbiol. 1990 May;136(5):965–973. doi: 10.1099/00221287-136-5-965. [DOI] [PubMed] [Google Scholar]
- Tsuyuguchi I., Kawasumi H., Takashima T., Tsuyuguchi T., Kishimoto S. Mycobacterium avium-Mycobacterium intracellular complex-induced suppression of T-cell proliferation in vitro by regulation of monocyte accessory cell activity. Infect Immun. 1990 May;58(5):1369–1378. doi: 10.1128/iai.58.5.1369-1378.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valone S. E., Rich E. A., Wallis R. S., Ellner J. J. Expression of tumor necrosis factor in vitro by human mononuclear phagocytes stimulated with whole Mycobacterium bovis BCG and mycobacterial antigens. Infect Immun. 1988 Dec;56(12):3313–3315. doi: 10.1128/iai.56.12.3313-3315.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadee A. A., Mendelsohn D., Rabson A. R. Characterization of a suppressor cell-activating factor (SCAF) released by adherent cells treated with M. tuberculosis. J Immunol. 1983 May;130(5):2266–2270. [PubMed] [Google Scholar]
- Wadee A. A., Sher R., Rabson A. R. Production of a suppressor factor by human adherent cells treated with mycobacteria. J Immunol. 1980 Sep;125(3):1380–1386. [PubMed] [Google Scholar]
- Watson G. A., Fu Y. X., Lopez D. M. Splenic macrophages from tumor-bearing mice co-expressing MAC-1 and MAC-2 antigens exert immunoregulatory functions via two distinct mechanisms. J Leukoc Biol. 1991 Feb;49(2):126–138. doi: 10.1002/jlb.49.2.126. [DOI] [PubMed] [Google Scholar]
- Woodbury J. L., Barrow W. W. Radiolabelling of Mycobacterium avium oligosaccharide determinant and use in macrophage studies. J Gen Microbiol. 1989 Jul;135(7):1875–1884. doi: 10.1099/00221287-135-7-1875. [DOI] [PubMed] [Google Scholar]
- Wright E. L., Barrow W. W. Inhibition of glycopeptidolipid synthesis resulting from treatment of Mycobacterium avium with 2-deoxy-D-glucose. Res Microbiol. 1991 Jun;142(5):597–608. doi: 10.1016/0923-2508(91)90193-e. [DOI] [PubMed] [Google Scholar]