Abstract
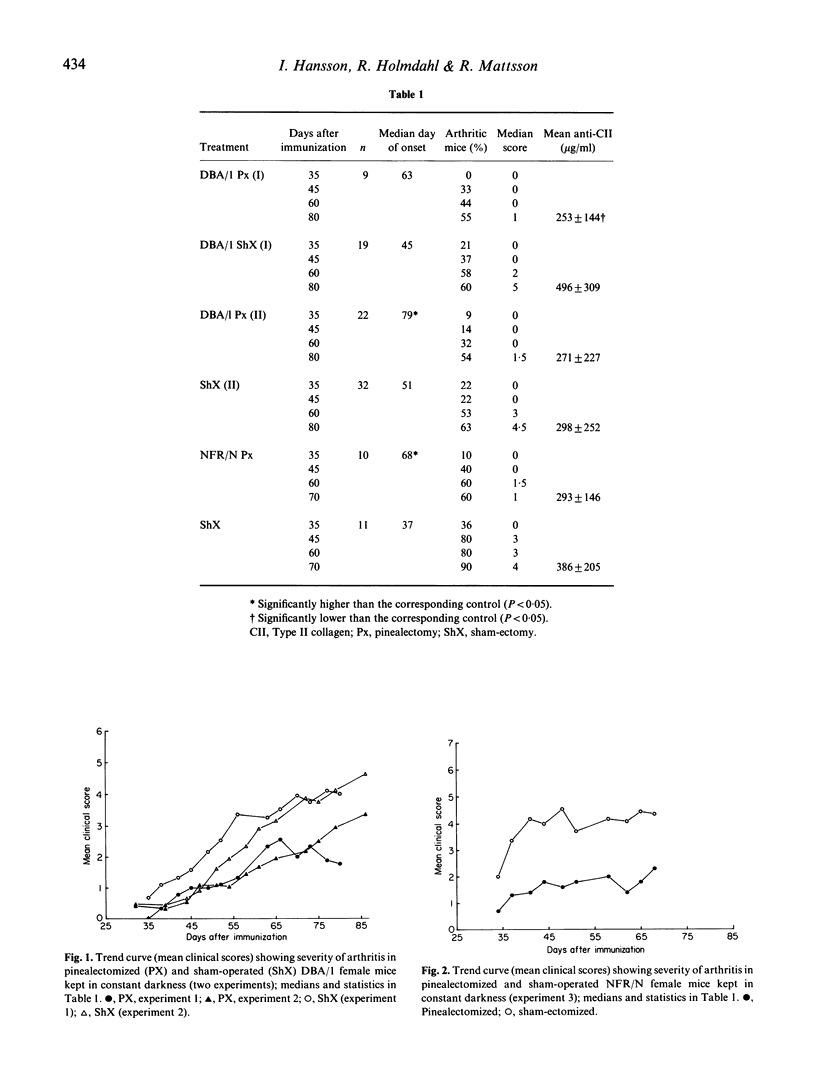
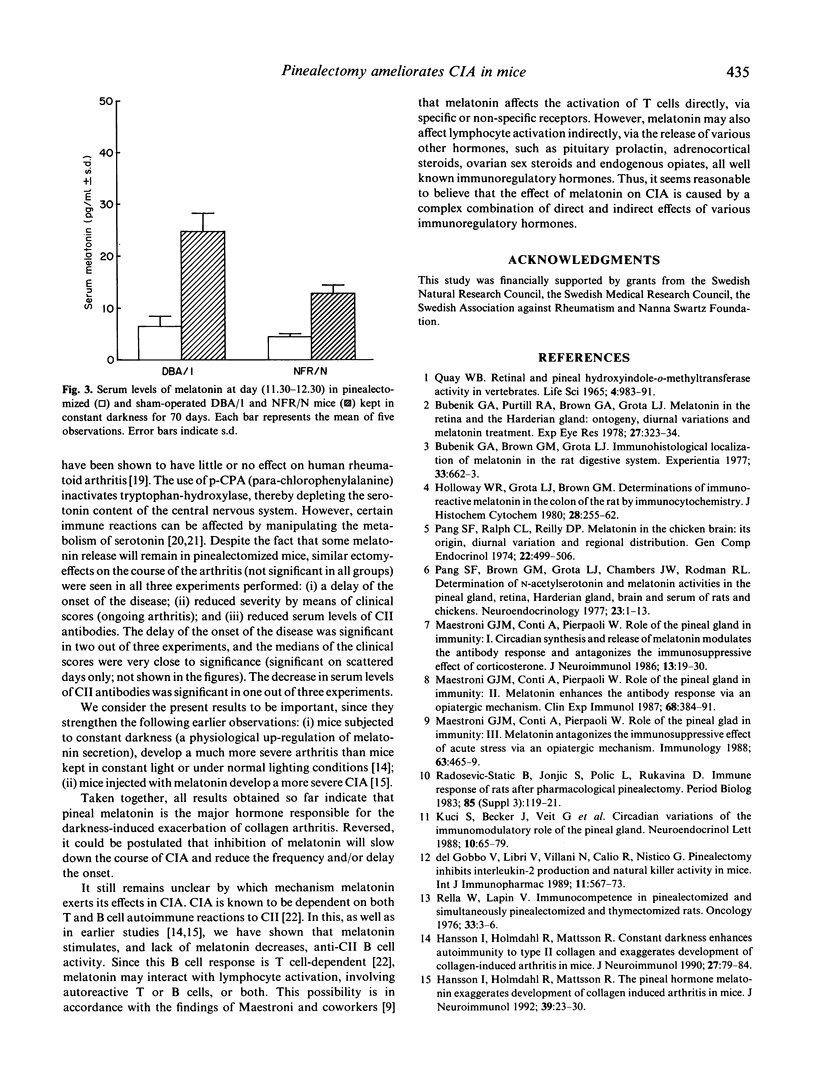
To extend our previous findings that exposure to constant darkness (stimulation of endogenous melatonin release) as well as treatment with exogenous melatonin magnifies the severity of collagen-induced arthritis in mice, we have examined the effects of melatonin cutback by removing the pineal gland. Two strains of mice, DBA/1 and NFR/N, were subjected to surgical pinealectomy. The melatonin levels in sera were reduced by approximately 70% by the pinealectomy compared with the corresponding sham-operated controls. After 3-4 weeks of rest the mice were immunized with rat type II collagen to induce autoimmune arthritis, and the animals were kept in constant darkness during the experiments. In comparison with the controls, all groups of pinealectomized mice showed reduced severity of the arthritis by means of (i) a slower onset of the disease, (ii) a less severe course of the disease (reduced clinical scores), and (iii) reduced serum levels of anti-collagen II antibodies. These effects were not significant in all experiments, but the trends were always the same. Thus, the present result strengthen the hypothesis that high physiological levels of melatonin (which can be induced by exposure to darkness) stimulate the immune system and cause exacerbation of autoimmune collagen II arthritis, while inhibition of melatonin release (pinealectomy or exposure to light) has a beneficial effect.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R., Anderson I. F., Oosthuizen R., Theron A. Immunological assessment of patients with rheumatoid arthritis--evaluation of the effects of propranolol. S Afr Med J. 1981 May 2;59(19):666–669. [PubMed] [Google Scholar]
- Boranić M., Pericić D., Poljak-Blazi M., Sverko V. Suppression of the immune response by drugs interfering with the metabolism of serotonin. Experientia. 1984 Oct 15;40(10):1153–1155. doi: 10.1007/BF01971469. [DOI] [PubMed] [Google Scholar]
- Bubenik G. A., Brown G. M., Grota L. J. Immunohistological localization of melatonin in the rat digestive system. Experientia. 1977 May 15;33(5):662–663. doi: 10.1007/BF01946561. [DOI] [PubMed] [Google Scholar]
- Bubenik G. A., Purtill R. A., Brown G. M., Grota L. J. Melatonin in the retina and the Harderian gland. Ontogeny, diurnal variations and melatonin treatment. Exp Eye Res. 1978 Sep;27(3):323–333. doi: 10.1016/0014-4835(78)90166-5. [DOI] [PubMed] [Google Scholar]
- Devoino L., Eliseeva L., Eremina O., Idova G., Cheido M. 5-Hydroxytryptophan effect on the development of the immune response: IgM and IgG antibodies and rosette formation in primary and secondary responses. Eur J Immunol. 1976 Jun;5(6):394–399. doi: 10.1002/eji.1830050608. [DOI] [PubMed] [Google Scholar]
- Hansson I., Holmdahl R., Mattsson R. Constant darkness enhances autoimmunity to type II collagen and exaggerates development of collagen-induced arthritis in DBA/1 mice. J Neuroimmunol. 1990 Apr;27(1):79–84. doi: 10.1016/0165-5728(90)90139-e. [DOI] [PubMed] [Google Scholar]
- Hansson I., Holmdahl R., Mattsson R. The pineal hormone melatonin exaggerates development of collagen-induced arthritis in mice. J Neuroimmunol. 1992 Jul;39(1-2):23–30. doi: 10.1016/0165-5728(92)90171-g. [DOI] [PubMed] [Google Scholar]
- Holloway W. R., Grota L. J., Brown G. M. Determination of immunoreactive melatonin in the colon of the rat by immunocytochemistry. J Histochem Cytochem. 1980 Mar;28(3):255–262. doi: 10.1177/28.3.6444434. [DOI] [PubMed] [Google Scholar]
- Holmdahl R., Andersson M., Goldschmidt T. J., Gustafsson K., Jansson L., Mo J. A. Type II collagen autoimmunity in animals and provocations leading to arthritis. Immunol Rev. 1990 Dec;118:193–232. doi: 10.1111/j.1600-065x.1990.tb00817.x. [DOI] [PubMed] [Google Scholar]
- Holmdahl R., Jansson L., Larsson E., Rubin K., Klareskog L. Homologous type II collagen induces chronic and progressive arthritis in mice. Arthritis Rheum. 1986 Jan;29(1):106–113. doi: 10.1002/art.1780290114. [DOI] [PubMed] [Google Scholar]
- Maestroni G. J., Conti A., Pierpaoli W. Role of the pineal gland in immunity. Circadian synthesis and release of melatonin modulates the antibody response and antagonizes the immunosuppressive effect of corticosterone. J Neuroimmunol. 1986 Nov;13(1):19–30. doi: 10.1016/0165-5728(86)90047-0. [DOI] [PubMed] [Google Scholar]
- Maestroni G. J., Conti A., Pierpaoli W. Role of the pineal gland in immunity. III. Melatonin antagonizes the immunosuppressive effect of acute stress via an opiatergic mechanism. Immunology. 1988 Mar;63(3):465–469. [PMC free article] [PubMed] [Google Scholar]
- Maestroni G. J., Conti A., Pierpaoli W. Role of the pineal gland in immunity: II. Melatonin enhances the antibody response via an opiatergic mechanism. Clin Exp Immunol. 1987 May;68(2):384–391. [PMC free article] [PubMed] [Google Scholar]
- Pang S. F., Brown G. M., Grota L. J., Chambers J. W., Rodman R. L. Determination of N-acetylserotonin and melatonin activities in the pineal gland, retina, harderian gland, brain and serum of rats and chickens. Neuroendocrinology. 1977;23(1):1–13. doi: 10.1159/000122649. [DOI] [PubMed] [Google Scholar]
- Pang S. F., Ralph C. L., Reilly D. P. Melatonin in the chicken brain: its origin, diurnal variation, and regional distribution. Gen Comp Endocrinol. 1974 Apr;22(4):499–506. doi: 10.1016/0016-6480(74)90026-4. [DOI] [PubMed] [Google Scholar]
- Quay W. B. Retinal and pineal hydroxyindole-o-methyl transferase activity in vertebrates. Life Sci. 1965 May;4(9):983–991. doi: 10.1016/0024-3205(65)90202-x. [DOI] [PubMed] [Google Scholar]
- Rella W., Lapin V. Immunocompetence of pinealectomized and simultaneously pinealectomized and thymectomized rats. Oncology. 1976;33(1):3–6. doi: 10.1159/000225091. [DOI] [PubMed] [Google Scholar]
- Smith B. D., Martin G. R., Miller E. J., Dorfman A., Swarm R. Nature of the collagen synthesized by a transplanted chondrosarcoma. Arch Biochem Biophys. 1975 Jan;166(1):181–186. doi: 10.1016/0003-9861(75)90378-1. [DOI] [PubMed] [Google Scholar]
- del Gobbo V., Libri V., Villani N., Caliò R., Nisticò G. Pinealectomy inhibits interleukin-2 production and natural killer activity in mice. Int J Immunopharmacol. 1989;11(5):567–573. doi: 10.1016/0192-0561(89)90187-2. [DOI] [PubMed] [Google Scholar]


