Abstract
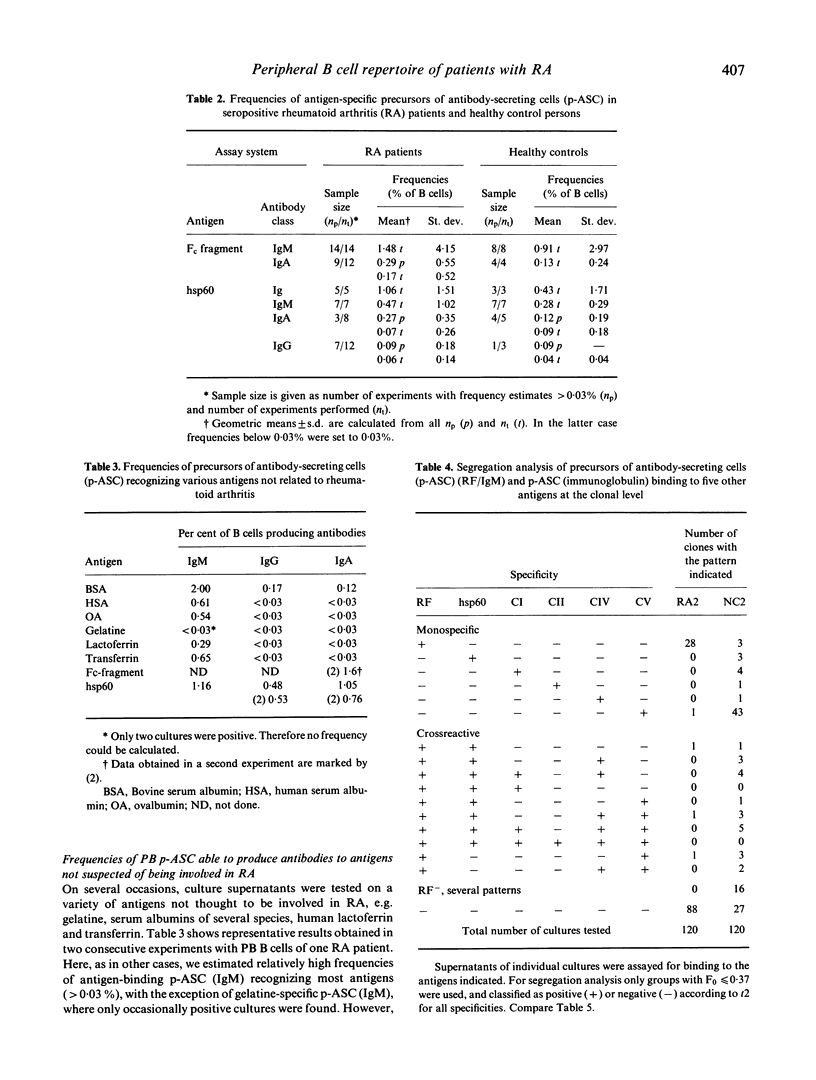
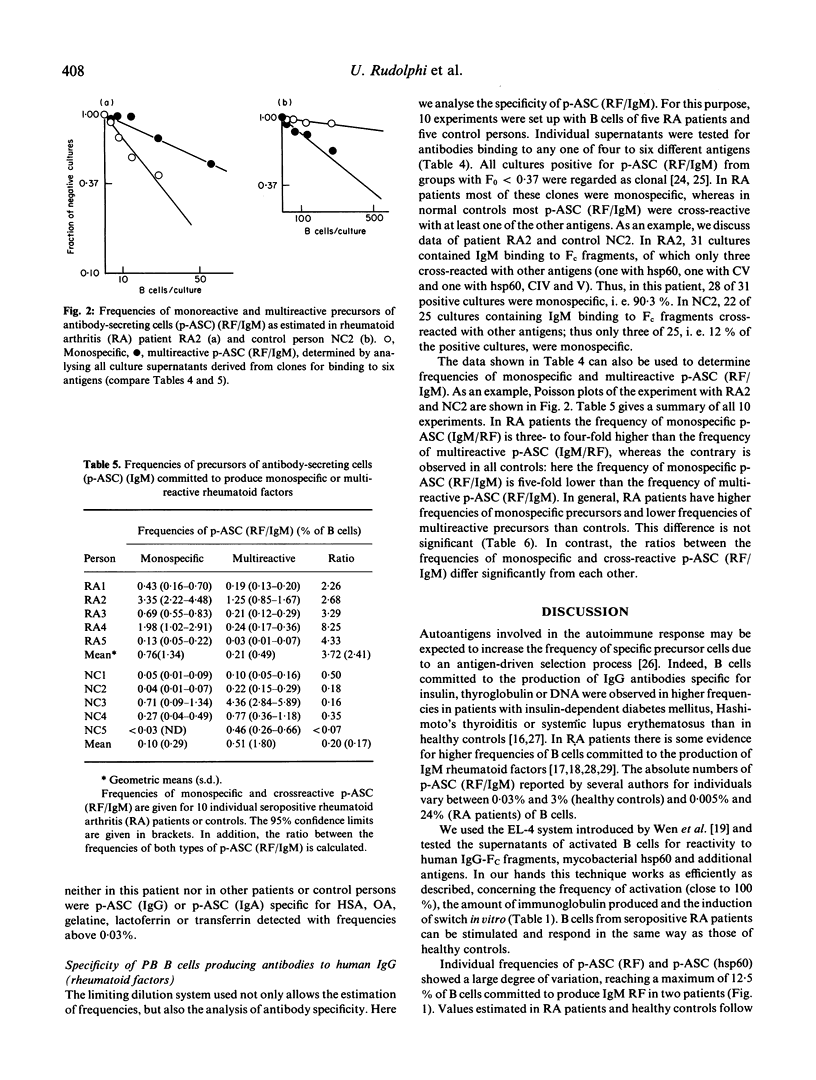
Using a potent in vitro limiting dilution culture system, we have activated human peripheral blood B cells to proliferate and to differentiate into antibody-secreting cells (ASC). Under these conditions 25-100% of B cells are clonally expanded and produce IgM, IgG or IgA. Culture supernatants were tested for antibodies binding to human IgG-Fc fragments (RF), the 65-kD heat shock protein of Mycobacterium bovis (hsp60), human collagens type I, II, IV, V, transferrin, lactoferrin, albumins, and gelatine. All blood samples contained precursors of ASC (p-ASC) able to produce IgM binding to these antigens in frequencies above 0.03% of B cells. Most interestingly, a significant difference exists between rheumatoid arthritis (RA) patients and controls, concerning the relative frequencies of p-ASC able to produce monospecific or multireactive RF. Whereas most p-ASC(RF) in RA patients are monospecific (mean ratio 3.7), most p-ASC(RF) in healthy control persons are cross-reactive with at least one of five other antigens tested (mean ratio 0.2). The data suggest a disease-specific expansion of p-ASC committed to the production of monospecific rheumatoid factors.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avrameas S. Natural autoantibodies: from 'horror autotoxicus' to 'gnothi seauton'. Immunol Today. 1991 May;12(5):154–159. doi: 10.1016/S0167-5699(05)80045-3. [DOI] [PubMed] [Google Scholar]
- Bahr G. M., Rook G. A., al-Saffar M., Van Embden J., Stanford J. L., Behbehani K. Antibody levels to mycobacteria in relation to HLA type: evidence for non-HLA-linked high levels of antibody to the 65 kD heat shock protein of M. bovis in rheumatoid arthritis. Clin Exp Immunol. 1988 Nov;74(2):211–215. [PMC free article] [PubMed] [Google Scholar]
- Bandeira A., Coutinho A., Martinez C., Pereira P. The origin of "natural antibodies" and the internal activity in the immune system. Int Rev Immunol. 1988 Mar;3(1-2):47–58. doi: 10.3109/08830188809051181. [DOI] [PubMed] [Google Scholar]
- Burastero S. E., Casali P., Wilder R. L., Notkins A. L. Monoreactive high affinity and polyreactive low affinity rheumatoid factors are produced by CD5+ B cells from patients with rheumatoid arthritis. J Exp Med. 1988 Dec 1;168(6):1979–1992. doi: 10.1084/jem.168.6.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burastero S. E., Cutolo M., Dessì V., Celada F. Monoreactive and polyreactive rheumatoid factors produced by in vitro Epstein-Barr virus-transformed peripheral blood and synovial B lymphocytes from rheumatoid arthritis patients. Scand J Immunol. 1990 Oct;32(4):347–357. doi: 10.1111/j.1365-3083.1990.tb02929.x. [DOI] [PubMed] [Google Scholar]
- Burmester G. R., Yu D. T., Irani A. M., Kunkel H. G., Winchester R. J. Ia+ T cells in synovial fluid and tissues of patients with rheumatoid arthritis. Arthritis Rheum. 1981 Nov;24(11):1370–1376. doi: 10.1002/art.1780241106. [DOI] [PubMed] [Google Scholar]
- Carson D. A., Chen P. P., Kipps T. J. New roles for rheumatoid factor. J Clin Invest. 1991 Feb;87(2):379–383. doi: 10.1172/JCI115007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casali P., Nakamura M., Ginsberg-Fellner F., Notkins A. L. Frequency of B cells committed to the production of antibodies to insulin in newly diagnosed patients with insulin-dependent diabetes mellitus and generation of high affinity human monoclonal IgG to insulin. J Immunol. 1990 May 15;144(10):3741–3747. [PubMed] [Google Scholar]
- Cohen I. R. Autoimmunity to chaperonins in the pathogenesis of arthritis and diabetes. Annu Rev Immunol. 1991;9:567–589. doi: 10.1146/annurev.iy.09.040191.003031. [DOI] [PubMed] [Google Scholar]
- Egeland T., Lea T., Saari G., Mellbye O. J., Natvig J. B. Quantitation of cells secreting rheumatoid factor of IgG, IgA, and IgM class after elution from rheumatoid synovial tissue. Arthritis Rheum. 1982 Dec;25(12):1445–1450. doi: 10.1002/art.1780251210. [DOI] [PubMed] [Google Scholar]
- Hirohata S., Inoue T., Miyamoto T. Frequency analysis of human peripheral blood B cells producing IgM-rheumatoid factor. Differential effects of stimulation with monoclonal antibodies to CD3 and Staphylococcus aureus. J Immunol. 1990 Sep 15;145(6):1681–1686. [PubMed] [Google Scholar]
- Karlsson-Parra A., Söderström K., Ferm M., Ivanyi J., Kiessling R., Klareskog L. Presence of human 65 kD heat shock protein (hsp) in inflamed joints and subcutaneous nodules of RA patients. Scand J Immunol. 1990 Jun;31(6):283–288. doi: 10.1111/j.1365-3083.1990.tb02770.x. [DOI] [PubMed] [Google Scholar]
- Klareskog L., Olsson T. Autoimmunity to collagen II and myelin basic protein: comparative studies in humans and rodents. Immunol Rev. 1990 Dec;118:285–310. doi: 10.1111/j.1600-065x.1990.tb00820.x. [DOI] [PubMed] [Google Scholar]
- Koga T., Wand-Württenberger A., DeBruyn J., Munk M. E., Schoel B., Kaufmann S. H. T cells against a bacterial heat shock protein recognize stressed macrophages. Science. 1989 Sep 8;245(4922):1112–1115. doi: 10.1126/science.2788923. [DOI] [PubMed] [Google Scholar]
- Melchers I., Fey K., Eichmann K. Quantitative studies on T cell diversity. III. Limiting dilution analysis of precursor cells for T helper cells reactive to xenogeneic erythrocytes. J Exp Med. 1982 Dec 1;156(6):1587–1603. doi: 10.1084/jem.156.6.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M., Burastero S. E., Ueki Y., Larrick J. W., Notkins A. L., Casali P. Probing the normal and autoimmune B cell repertoire with Epstein-Barr virus. Frequency of B cells producing monoreactive high affinity autoantibodies in patients with Hashimoto's disease and systemic lupus erythematosus. J Immunol. 1988 Dec 15;141(12):4165–4172. [PubMed] [Google Scholar]
- Nelson J. L., Nardella F. A., Oppliger I. R., Mannik M. Rheumatoid factors from patients with rheumatoid arthritis possess private repertoires of idiotypes. J Immunol. 1987 Mar 1;138(5):1391–1396. [PubMed] [Google Scholar]
- Olee T., Yang P. M., Siminovitch K. A., Olsen N. J., Hillson J., Wu J., Kozin F., Carson D. A., Chen P. P. Molecular basis of an autoantibody-associated restriction fragment length polymorphism that confers susceptibility to autoimmune diseases. J Clin Invest. 1991 Jul;88(1):193–203. doi: 10.1172/JCI115277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual V., Capra J. D. B-cell superantigens? Curr Biol. 1991 Oct;1(5):315–317. doi: 10.1016/0960-9822(91)90097-g. [DOI] [PubMed] [Google Scholar]
- Pascual V., Randen I., Thompson K., Sioud M., Forre O., Natvig J., Capra J. D. The complete nucleotide sequences of the heavy chain variable regions of six monospecific rheumatoid factors derived from Epstein-Barr virus-transformed B cells isolated from the synovial tissue of patients with rheumatoid arthritis. Further evidence that some autoantibodies are unmutated copies of germ line genes. J Clin Invest. 1990 Oct;86(4):1320–1328. doi: 10.1172/JCI114841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluschke G., Ricken G., Taube H., Kroninger S., Melchers I., Peter H. H., Eichmann K., Krawinkel U. Biased T cell receptor V alpha region repertoire in the synovial fluid of rheumatoid arthritis patients. Eur J Immunol. 1991 Nov;21(11):2749–2754. doi: 10.1002/eji.1830211115. [DOI] [PubMed] [Google Scholar]
- Randen I., Thompson K. M., Natvig J. B., Førre O., Waalen K. Human monoclonal rheumatoid factors derived from the polyclonal repertoire of rheumatoid synovial tissue: production and characterization. Clin Exp Immunol. 1989 Oct;78(1):13–18. [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Arguelles A., Presno-Bernal M. Demonstration of a cross-reactive idiotype (IdRQ) in rheumatoid factors from patients with rheumatoid arthritis but not in rheumatoid factors from healthy, aged subjects. Arthritis Rheum. 1989 Feb;32(2):134–138. doi: 10.1002/anr.1780320204. [DOI] [PubMed] [Google Scholar]
- Saizawa K. M., Melchers I., Eichmann K. Genetic control of B cell function. III. IgVH-controlled polymorphism in the frequencies of B cells that recognize xenogeneic red blood cells. Eur J Immunol. 1985 Feb;15(2):124–131. doi: 10.1002/eji.1830150205. [DOI] [PubMed] [Google Scholar]
- Sasso E. H., Silverman G. J., Mannik M. Human IgM molecules that bind staphylococcal protein A contain VHIII H chains. J Immunol. 1989 Apr 15;142(8):2778–2783. [PubMed] [Google Scholar]
- Shin E. K., Matsuda F., Nagaoka H., Fukita Y., Imai T., Yokoyama K., Soeda E., Honjo T. Physical map of the 3' region of the human immunoglobulin heavy chain locus: clustering of autoantibody-related variable segments in one haplotype. EMBO J. 1991 Dec;10(12):3641–3645. doi: 10.1002/j.1460-2075.1991.tb04930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stastny P. Association of the B-cell alloantigen DRw4 with rheumatoid arthritis. N Engl J Med. 1978 Apr 20;298(16):869–871. doi: 10.1056/NEJM197804202981602. [DOI] [PubMed] [Google Scholar]
- Stuart J. M., Townes A. S., Kang A. H. Collagen autoimmune arthritis. Annu Rev Immunol. 1984;2:199–218. doi: 10.1146/annurev.iy.02.040184.001215. [DOI] [PubMed] [Google Scholar]
- Tarkowski A., Klareskog L., Carlsten H., Herberts P., Koopman W. J. Secretion of antibodies to types I and II collagen by synovial tissue cells in patients with rheumatoid arthritis. Arthritis Rheum. 1989 Sep;32(9):1087–1092. doi: 10.1002/anr.1780320906. [DOI] [PubMed] [Google Scholar]
- Taswell C. Limiting dilution assays for the determination of immunocompetent cell frequencies. I. Data analysis. J Immunol. 1981 Apr;126(4):1614–1619. [PubMed] [Google Scholar]
- Tsoulfa G., Rook G. A., Van-Embden J. D., Young D. B., Mehlert A., Isenberg D. A., Hay F. C., Lydyard P. M. Raised serum IgG and IgA antibodies to mycobacterial antigens in rheumatoid arthritis. Ann Rheum Dis. 1989 Feb;48(2):118–123. doi: 10.1136/ard.48.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eden W. Heat-shock proteins in autoimmune arthritis: a critical contribution based on the adjuvant arthritis model. APMIS. 1990 May;98(5):383–394. doi: 10.1111/j.1699-0463.1990.tb01048.x. [DOI] [PubMed] [Google Scholar]
- Vischer T. L., Werner-Favre C. F., Wen L., Zubler R. H. Quantitative analysis of precursors frequency of rheumatoid factor (RF) producing human B cells. Scand J Rheumatol Suppl. 1988;75:123–126. doi: 10.3109/03009748809096752. [DOI] [PubMed] [Google Scholar]
- Wen L., Hanvanich M., Werner-Favre C., Brouwers N., Perrin L. H., Zubler R. H. Limiting dilution assay for human B cells based on their activation by mutant EL4 thymoma cells: total and antimalaria responder B cell frequencies. Eur J Immunol. 1987 Jun;17(6):887–892. doi: 10.1002/eji.1830170624. [DOI] [PubMed] [Google Scholar]
- Yan T., Burkhardt H., Ritter T., Bröker B., Mann K. H., Bertling W. M., von der Mark K., Emmrich F. Specificity and T cell receptor beta chain usage of a human collagen type II-reactive T cell clone derived from a healthy individual. Eur J Immunol. 1992 Jan;22(1):51–56. doi: 10.1002/eji.1830220109. [DOI] [PubMed] [Google Scholar]
- Yang P. M., Olsen N. J., Siminovitch K. A., Olee T., Kozin F., Carson D. A., Chen P. P. Possible deletion of a developmentally regulated heavy-chain variable region gene in autoimmune diseases. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7907–7911. doi: 10.1073/pnas.87.20.7907. [DOI] [PMC free article] [PubMed] [Google Scholar]


