Abstract
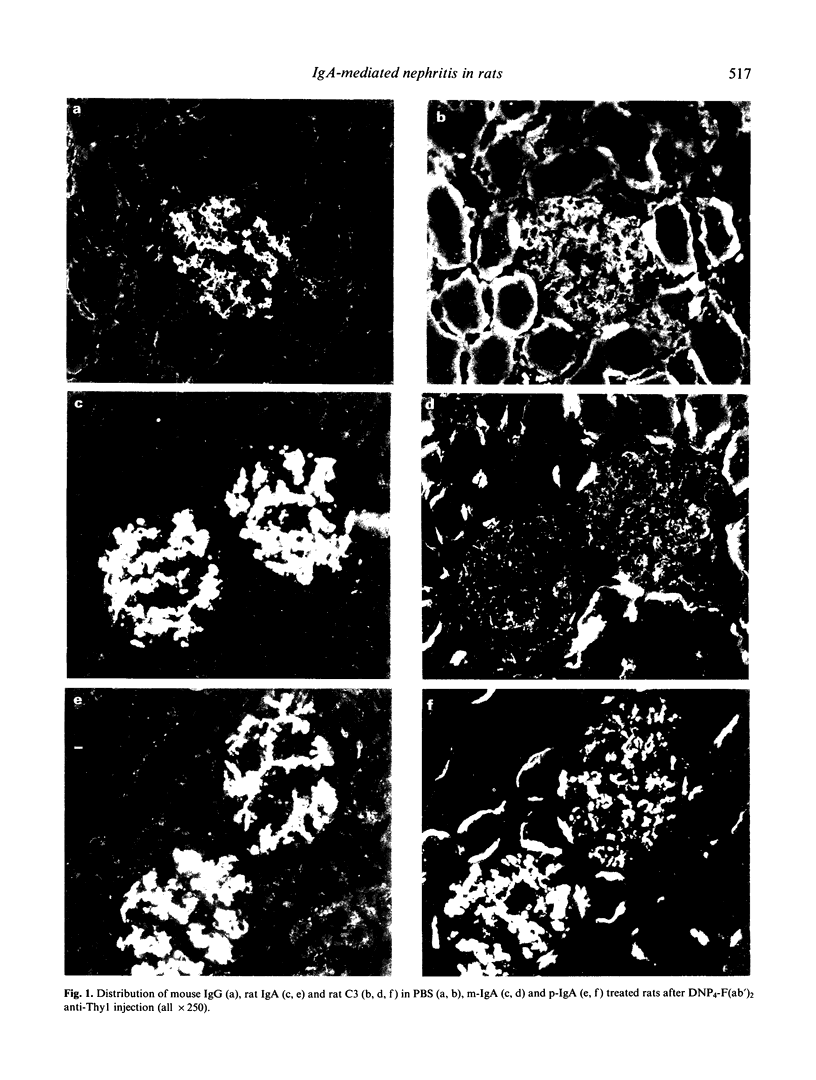
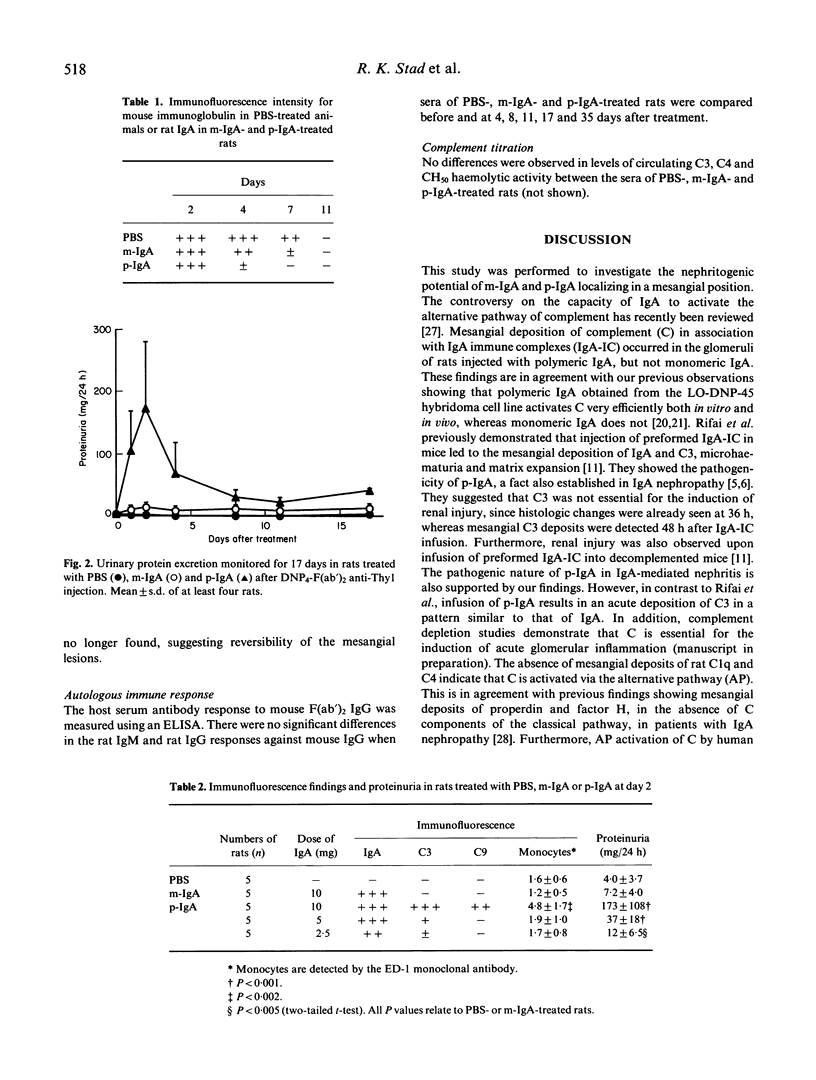
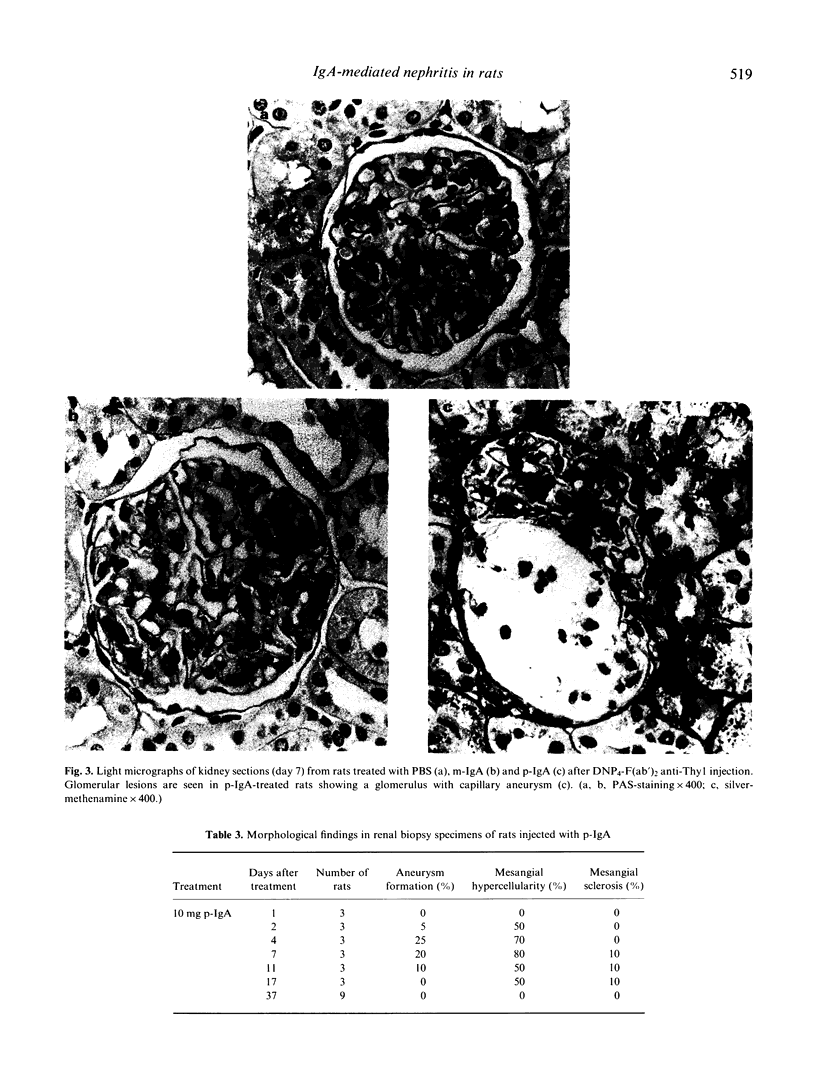
An acute model for IgA-mediated glomerular inflammation in rats was induced by the in situ deposition of IgA directly into the glomerular mesangium. F(ab')2 anti-Thy1 MoAb was used to anchor an antigen, DNP (2,4-dinitrophenol), in the glomeruli of rats. Subsequent infusion of rat polymeric (p-) or monomeric (m-) IgA MoAb with specificity for DNP resulted in mesangial deposition of IgA in both groups of rats. However, acute proteinuria was observed only in p-IgA-treated rats and not in PBS- or m-IgA-treated rats. Immunofluorescence analysis revealed deposition of C3 in an identical pattern to that of IgA in the glomeruli of p-IgA-treated rats. No mesangial deposits of C4 or C1q were seen in these animals. Rats receiving m-IgA or PBS displayed no detectable C3, C4 or C1q deposition. The amount of proteinuria in p-IgA-treated rats was related to the amount of deposited C3. The presence of intraglomerular monocytes was only observed 2 days after p-IgA injection. By light microscopy, aneurysm formation, mesangial hypercellularity and matrix expansion were seen only in p-IgA-treated rats. However, by 37 days post-injection complete resolution of the lesions was observed. No histological renal changes were observed in PBS- or m-IgA-treated rats. In conclusion, an acute form of IgA-mediated nephritis in rats was induced by p-IgA but not by m-IgA. This reproducible model provides a basis for further study into the mechanisms of IgA-mediated glomerular inflammation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagchus W. M., Hoedemaeker P. J., Rozing J., Bakker W. W. Acute glomerulonephritis after intravenous injection of monoclonal anti-thymocyte antibodies in the rat. Immunol Lett. 1986 Mar;12(2-3):109–113. doi: 10.1016/0165-2478(86)90091-x. [DOI] [PubMed] [Google Scholar]
- Bartolotti S. R., Peters D. K. Delayed removal of renal-bound antigen in decomplemented rabbits with acute serum sickness. Clin Exp Immunol. 1978 May;32(2):199–206. [PMC free article] [PubMed] [Google Scholar]
- Bene M. C., Faure G. C. Composition of mesangial deposits in IgA nephropathy: complement factors. Nephron. 1987;46(2):219–219. doi: 10.1159/000184350. [DOI] [PubMed] [Google Scholar]
- Bene M. C., Faure G., Duheille J. IgA nephropathy: characterization of the polymeric nature of mesangial deposits by in vitro binding of free secretory component. Clin Exp Immunol. 1982 Mar;47(3):527–534. [PMC free article] [PubMed] [Google Scholar]
- Berger J., Hinglais N. Les ddpôts intercapillaires d'IgA-IgG. J Urol Nephrol (Paris) 1968 Sep;74(9):694–695. [PubMed] [Google Scholar]
- Caulin-Glaser T., Gallo G. R., Lamm M. E. Nondissociating cationic immune complexes can deposit in glomerular basement membrane. J Exp Med. 1983 Nov 1;158(5):1561–1572. doi: 10.1084/jem.158.5.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley M. E., Delacroix D. L. Intravascular and mucosal immunoglobulin A: two separate but related systems of immune defense? Ann Intern Med. 1987 Jun;106(6):892–899. doi: 10.7326/0003-4819-106-6-892. [DOI] [PubMed] [Google Scholar]
- D'Amico G. The commonest glomerulonephritis in the world: IgA nephropathy. Q J Med. 1987 Sep;64(245):709–727. [PubMed] [Google Scholar]
- Dijkstra C. D., Döpp E. A., Joling P., Kraal G. The heterogeneity of mononuclear phagocytes in lymphoid organs: distinct macrophage subpopulations in the rat recognized by monoclonal antibodies ED1, ED2 and ED3. Immunology. 1985 Mar;54(3):589–599. [PMC free article] [PubMed] [Google Scholar]
- EISEN H. N. PREPARATION OF PURIFIED ANTI-2,4-DINITROPHENYL ANTIBODIES. Methods Med Res. 1964;10:94–102. [PubMed] [Google Scholar]
- Emancipator S. N., Gallo G. R., Lamm M. E. Experimental IgA nephropathy induced by oral immunization. J Exp Med. 1983 Feb 1;157(2):572–582. doi: 10.1084/jem.157.2.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emancipator S. N., Lamm M. E. IgA nephropathy: pathogenesis of the most common form of glomerulonephritis. Lab Invest. 1989 Feb;60(2):168–183. [PubMed] [Google Scholar]
- Emancipator S. N., Ovary Z., Lamm M. E. The role of mesangial complement in the hematuria of experimental IgA nephropathy. Lab Invest. 1987 Sep;57(3):269–276. [PubMed] [Google Scholar]
- Faille-Kuyber E. H., Kater L., Kooiker C. J., Dorhout Mees E. J. IgA-deposits in cutaneous blood-vessel walls and mesangium in Henoch-Schönlein syndrome. Lancet. 1973 Apr 21;1(7808):892–893. doi: 10.1016/s0140-6736(73)91471-2. [DOI] [PubMed] [Google Scholar]
- Furness P. N., Turner D. R. Chronic serum sickness glomerulonephritis: removal of glomerular antigen and electron-dense deposits is largely dependent on plasma complement. Clin Exp Immunol. 1988 Oct;74(1):126–130. [PMC free article] [PubMed] [Google Scholar]
- Gorter A., Hiemstra P. S., Klar-Mohamad N., van Es L. A., Daha M. R. Binding, internalization and degradation of soluble aggregates of human secretory IgA by resident rat peritoneal macrophages. Immunology. 1988 Aug;64(4):703–708. [PMC free article] [PubMed] [Google Scholar]
- Hiemstra P. S., Gorter A., Stuurman M. E., Van Es L. A., Daha M. R. Activation of the alternative pathway of complement by human serum IgA. Eur J Immunol. 1987 Mar;17(3):321–326. doi: 10.1002/eji.1830170304. [DOI] [PubMed] [Google Scholar]
- Imai H., Nakamoto Y., Asakura K., Miki K., Yasuda T., Miura A. B. Spontaneous glomerular IgA deposition in ddY mice: an animal model of IgA nephritis. Kidney Int. 1985 May;27(5):756–761. doi: 10.1038/ki.1985.76. [DOI] [PubMed] [Google Scholar]
- Isaacs K. L., Miller F. Role of antigen size and charge in immune complex glomerulonephritis. Lab Invest. 1982 Aug;47(2):198–205. [PubMed] [Google Scholar]
- Johnson K. J., Ward P. A., Kunkel R. G., Wilson B. S. Mediation of IgA induced lung injury in the rat. Role of macrophages and reactive oxygen products. Lab Invest. 1986 May;54(5):499–506. [PubMed] [Google Scholar]
- König W., Bitter-Suermann D., Dierich M., Limbert M., Schorlemmer H. U., Hadding U. DNP-antigens activate the alternate pathway of the complement system. J Immunol. 1974 Aug;113(2):501–506. [PubMed] [Google Scholar]
- Lamoyi E., Nisonoff A. Preparation of F(ab')2 fragments from mouse IgG of various subclasses. J Immunol Methods. 1983 Jan 28;56(2):235–243. doi: 10.1016/0022-1759(83)90415-5. [DOI] [PubMed] [Google Scholar]
- Lucisano Valim Y. M., Lachmann P. J. The effect of antibody isotype and antigenic epitope density on the complement-fixing activity of immune complexes: a systematic study using chimaeric anti-NIP antibodies with human Fc regions. Clin Exp Immunol. 1991 Apr;84(1):1–8. doi: 10.1111/j.1365-2249.1991.tb08115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melvin T., Burke B., Michael A. F., Kim Y. Experimental IgA nephropathy in bile duct ligated rats. Clin Immunol Immunopathol. 1983 Jun;27(3):369–377. doi: 10.1016/0090-1229(83)90089-2. [DOI] [PubMed] [Google Scholar]
- Mené P., Simonson M. S., Dunn M. J. Physiology of the mesangial cell. Physiol Rev. 1989 Oct;69(4):1347–1424. doi: 10.1152/physrev.1989.69.4.1347. [DOI] [PubMed] [Google Scholar]
- Nolasco F. E., Cameron J. S., Hartley B., Coelho A., Hildreth G., Reuben R. Intraglomerular T cells and monocytes in nephritis: study with monoclonal antibodies. Kidney Int. 1987 May;31(5):1160–1166. doi: 10.1038/ki.1987.123. [DOI] [PubMed] [Google Scholar]
- Oite T., Batsford S. R., Mihatsch M. J., Takamiya H., Vogt A. Quantitative studies of in situ immune complex glomerulonephritis in the rat induced by planted, cationized antigen. J Exp Med. 1982 Feb 1;155(2):460–474. doi: 10.1084/jem.155.2.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portis J. L., Coe J. E. Deposition of IgA in renal glomeruli of mink affected with Aleutian disease. Am J Pathol. 1979 Jul;96(1):227–236. [PMC free article] [PubMed] [Google Scholar]
- Rauterberg E. W., Lieberknecht H. M., Wingen A. M., Ritz E. Complement membrane attack (MAC) in idiopathic IgA-glomerulonephritis. Kidney Int. 1987 Mar;31(3):820–829. doi: 10.1038/ki.1987.72. [DOI] [PubMed] [Google Scholar]
- Rifai A., Chen A., Imai H. Complement activation in experimental IgA nephropathy: an antigen-mediated process. Kidney Int. 1987 Dec;32(6):838–844. doi: 10.1038/ki.1987.284. [DOI] [PubMed] [Google Scholar]
- Rifai A., Small P. A., Jr, Teague P. O., Ayoub E. M. Experimental IgA nephropathy. J Exp Med. 1979 Nov 1;150(5):1161–1173. doi: 10.1084/jem.150.5.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rits M., Cormont F., Bazin H., Meykens R., Vaerman J. P. Rat monoclonal antibodies. VI. Production of IgA secreting hybridomas with specificity for the 2,4-dinitrophenyl (DNP) hapten. J Immunol Methods. 1986 May 1;89(1):81–87. doi: 10.1016/0022-1759(86)90034-7. [DOI] [PubMed] [Google Scholar]
- Rits M., Hiemstra P. S., Bazin H., Van Es L. A., Vaerman J. P., Daha M. R. Activation of rat complement by soluble and insoluble rat IgA immune complexes. Eur J Immunol. 1988 Dec;18(12):1873–1880. doi: 10.1002/eji.1830181202. [DOI] [PubMed] [Google Scholar]
- Stad R. K., Bogers W. M., Muizert Y., van Es L. A., Daha M. R. Deposition of IgA is associated with macrophage influx in the kidney of rats. Scand J Immunol. 1991 Jul;34(1):81–89. doi: 10.1111/j.1365-3083.1991.tb01523.x. [DOI] [PubMed] [Google Scholar]
- Stad R. K., Bogers W. M., Thoomes-van der Sluys M. E., Van Es L. A., Daha M. R. In vivo activation of complement by IgA in a rat model. Clin Exp Immunol. 1992 Jan;87(1):138–143. doi: 10.1111/j.1365-2249.1992.tb06427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syré G. IGA mesangial glomerulonephritis; significance and pathogenesis of segmental-focal glomerular lesions. Virchows Arch A Pathol Anat Histopathol. 1983;402(1):11–24. doi: 10.1007/BF00695045. [DOI] [PubMed] [Google Scholar]
- Valentijn R. M., Radl J., Haaijman J. J., Vermeer B. J., Weening J. J., Kauffmann R. H., Daha M. R., van Es L. A. Circulating and mesangial secretory component-binding IgA-1 in primary IgA nephropathy. Kidney Int. 1984 Nov;26(5):760–766. doi: 10.1038/ki.1984.213. [DOI] [PubMed] [Google Scholar]
- Williams A. F., Galfrè G., Milstein C. Analysis of cell surfaces by xenogeneic myeloma-hybrid antibodies: differentiation antigens of rat lymphocytes. Cell. 1977 Nov;12(3):663–673. doi: 10.1016/0092-8674(77)90266-5. [DOI] [PubMed] [Google Scholar]
- Wyatt R. J., Kanayama Y., Julian B. A., Negoro N., Sugimoto S., Hudson E. C., Curd J. G. Complement activation in IgA nephropathy. Kidney Int. 1987 Apr;31(4):1019–1023. doi: 10.1038/ki.1987.101. [DOI] [PubMed] [Google Scholar]
- Yoshioka K., Takemura T., Akano N., Okada M., Yagi K., Maki S., Inai S., Akita H., Koitabashi Y., Takekoshi Y. IgA nephropathy in patients with congenital C9 deficiency. Kidney Int. 1992 Nov;42(5):1253–1258. doi: 10.1038/ki.1992.412. [DOI] [PubMed] [Google Scholar]
- Yoshioka K., Takemura T., Aya N., Akano N., Miyamoto H., Maki S. Monocyte infiltration and cross-linked fibrin deposition in IgA nephritis and Henoch-Schoenlein purpura nephritis. Clin Nephrol. 1989 Sep;32(3):107–112. [PubMed] [Google Scholar]
- van Es L. A. Pathogenesis of IgA nephropathy. Kidney Int. 1992 Jun;41(6):1720–1729. doi: 10.1038/ki.1992.246. [DOI] [PubMed] [Google Scholar]