Abstract
An analysis of the relationship between the immune response to ubiquitous herpes family viruses, namely Epstein-Barr virus (EBV), cytomegalovirus (CMV), and varicella-zoster virus (VZV) and the presence of rheumatoid factors (RF), which are autoantibodies characteristic of patients with rheumatoid arthritis (RA), was conducted. Antibody profiles (RF, anti-viral antibodies) were monitored in the serum of the RA patients, and in normal individuals. No patient was found to have circulating RF in the absence of anti-viral antibodies. When the patients and normal controls were subdivided according to the presence of serum RF, it was found that when RF were present, the frequency of anti-CMV antibodies, but not anti-EBV or anti-VZV antibodies, was significantly higher (P = 0.02) when compared with RF-negative individuals. The titres of anti-CMV but not anti-VZV antibodies were found to increase in the RA patients with disease duration. To see if these viruses could stimulate RF production in vitro, peripheral blood mononuclear cells (PBMC) isolated from the patients and normal controls were stimulated with viral antigens. PBMC from normal controls, but not from RA patients, appeared to be responsive to viral antigen stimulation and produced RF. These data suggest that the immune response to CMV, to a greater extent than to EBV or VZV, correlates with the presence of RF.
Full text
PDF

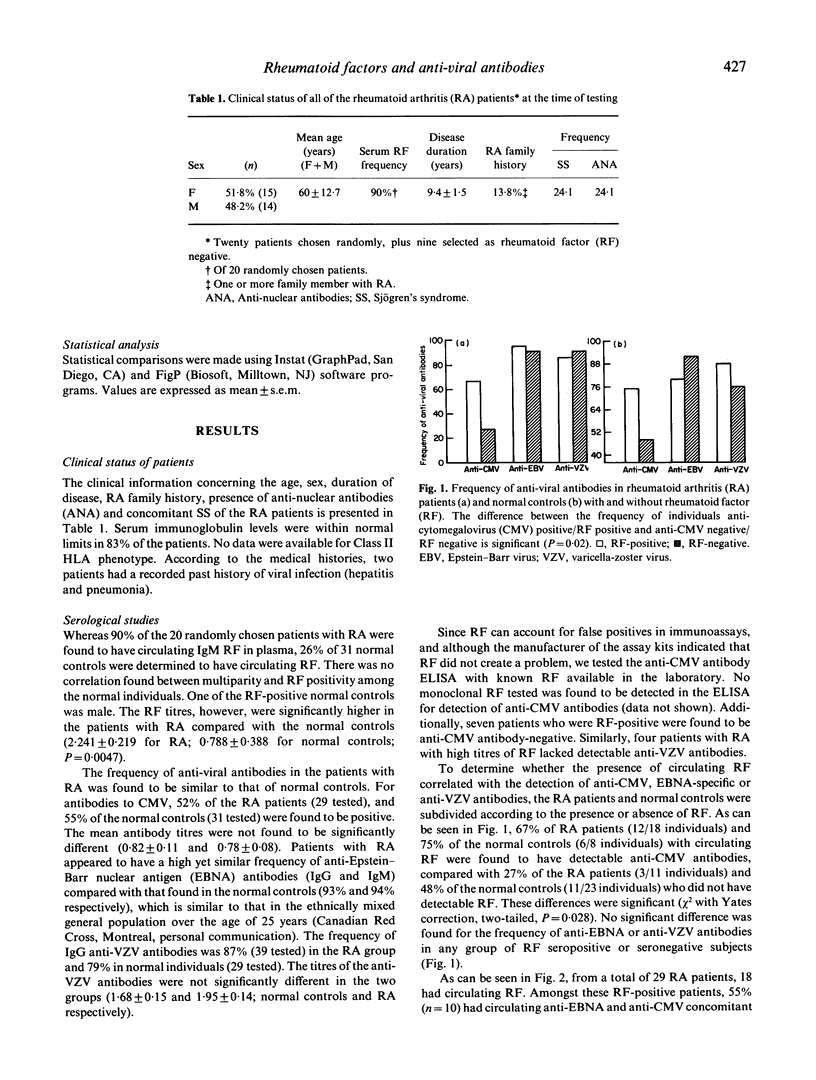
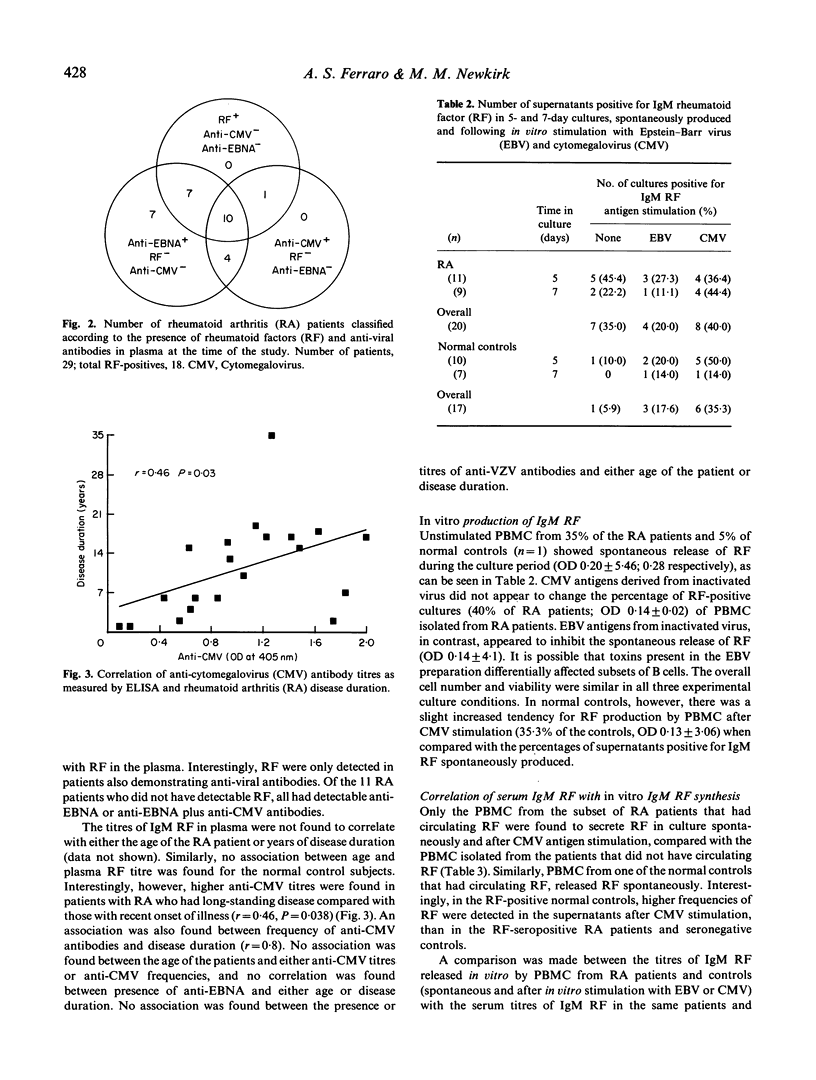



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alspaugh M. A., Henle G., Lennette E. T., Henle W. Elevated levels of antibodies to Epstein-Barr virus antigens in sera and synovial fluids of patients with rheumatoid arthritis. J Clin Invest. 1981 Apr;67(4):1134–1140. doi: 10.1172/JCI110127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alspaugh M. A., Jensen F. C., Rabin H., Tan E. M. Lymphocytes transformed by Epstein-Barr virus. Induction of nuclear antigen reactive with antibody in rheumatoid arthritis. J Exp Med. 1978 Apr 1;147(4):1018–1027. doi: 10.1084/jem.147.4.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Baldwin W. M., 3rd, Westedt M. L., van Gemert G. W., Henny F. C., Paul L. C., Daha M. R., van Es L. A. Association of rheumatoid factors in renal transplant recipients with cytomegalovirus infection and not with rejection. Transplantation. 1987 May;43(5):658–662. doi: 10.1097/00007890-198705000-00011. [DOI] [PubMed] [Google Scholar]
- Bartfeld H. Distribution of rheumatoid factor activity in nonrheumatoid states. Ann N Y Acad Sci. 1969 Dec 10;168(1):30–40. doi: 10.1111/j.1749-6632.1969.tb43092.x. [DOI] [PubMed] [Google Scholar]
- Bluestone R., Goldberg L. S. Effect of D-penicillamine on serum immunoglobulins and rheumatoid factor. Ann Rheum Dis. 1973 Jan;32(1):50–52. doi: 10.1136/ard.32.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun R. W., Reiser H. C. Replication of human cytomegalovirus in human peripheral blood T cells. J Virol. 1986 Oct;60(1):29–36. doi: 10.1128/jvi.60.1.29-36.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalano M. A., Carson D. A., Niederman J. C., Feorino P., Vaughan J. H. Antibody to the rheumatoid arthritis nuclear antigen. Its relationship to in vivo Epstein-Barr virus infection. J Clin Invest. 1980 May;65(5):1238–1242. doi: 10.1172/JCI109779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. I., Corey G. R. Cytomegalovirus infection in the normal host. Medicine (Baltimore) 1985 Mar;64(2):100–114. doi: 10.1097/00005792-198503000-00003. [DOI] [PubMed] [Google Scholar]
- Depper J. M., Bluestein H. G., Zvaifler N. J. Impaired regulation of Epstein-Barr virus-induced lymphocyte proliferation in rheumatoid arthritis is due to a T cell defect. J Immunol. 1981 Nov;127(5):1899–1902. [PubMed] [Google Scholar]
- FRANKLIN E. C., HOLMAN H. R., MULLER-EBERHARD H. J., KUNKEL H. G. An unusual protein component of high molecular weight in the serum of certain patients with rheumatoid arthritis. J Exp Med. 1957 May 1;105(5):425–438. doi: 10.1084/jem.105.5.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrell P. B., Aitcheson C. T., Pearson G. R., Tan E. M. Seroepidemiological study of relationships between Epstein-Barr virus and rheumatoid arthritis. J Clin Invest. 1981 Mar;67(3):681–687. doi: 10.1172/JCI110083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman H. M., Pincus T., Gibilisco P., Baker D., Glazer J. P., Plotkin S. A., Schumacher H. R. Acute monoarticular arthritis caused by herpes simplex virus and cytomegalovirus. Am J Med. 1980 Aug;69(2):241–247. doi: 10.1016/0002-9343(80)90384-8. [DOI] [PubMed] [Google Scholar]
- Grahame R., Armstrong R., Simmons N. A., Mims C. A., Wilton J. M., Laurent R. Isolation of rubella virus from synovial fluid in five cases of seronegative arthritis. Lancet. 1981 Sep 26;2(8248):649–651. doi: 10.1016/s0140-6736(81)90993-4. [DOI] [PubMed] [Google Scholar]
- Hamerman D., Gresser I., Smith C. Isolation of cytomegalovirus from synovial cells of a patient with rheumatoid arthritis. J Rheumatol. 1982 Sep-Oct;9(5):658–664. [PubMed] [Google Scholar]
- Johnson P. M., Faulk W. P. Rheumatoid factor: its nature, specificity, and production in rheumatoid arthritis. Clin Immunol Immunopathol. 1976 Nov;6(3):414–430. doi: 10.1016/0090-1229(76)90094-5. [DOI] [PubMed] [Google Scholar]
- KUNKEL H. G., MULLER-EBERHARD H. J., FUDENBERG H. H., TOMASI T. B. Gamma globulin complexes in rheumatoid arthritis and certain other conditions. J Clin Invest. 1961 Jan;40:117–129. doi: 10.1172/JCI104224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalliomäki J. L., Halonen P., Salmi A. Virus antibodies in serum and synovial fluid of patients with rheumatoid arthritis and other connective tissue diseases. Ann Rheum Dis. 1975 Feb;34(1):43–48. doi: 10.1136/ard.34.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopman W. J., Schrohenloher R. E. Enhanced in vitro synthesis of IgM rheumatoid factor in rheumatoid arthritis. Arthritis Rheum. 1980 Sep;23(9):985–992. doi: 10.1002/art.1780230904. [DOI] [PubMed] [Google Scholar]
- Korbet S. M., Schwartz M. M., Lewis E. J. Immune complex deposition and coronary vasculitis in systemic lupus erythematosus. Report of two cases. Am J Med. 1984 Jul;77(1):141–146. doi: 10.1016/0002-9343(84)90449-2. [DOI] [PubMed] [Google Scholar]
- Levinson A. I., Tar L. In vitro IgM rheumatoid-factor production induced by tetanus toxoid. J Allergy Clin Immunol. 1988 Apr;81(4):730–736. doi: 10.1016/0091-6749(88)91046-9. [DOI] [PubMed] [Google Scholar]
- Levy R. J., Haidar M., Park H., Tar L., Levinson A. I. Bacterial peptidoglycan induces in vitro rheumatoid factor production by lymphocytes of healthy subjects. Clin Exp Immunol. 1986 May;64(2):311–317. [PMC free article] [PubMed] [Google Scholar]
- Male D., Young A., Pilkington C., Sutherland S., Roitt I. M. Antibodies to EB virus- and cytomegalovirus-induced antigens in early rheumatoid disease. Clin Exp Immunol. 1982 Nov;50(2):341–346. [PMC free article] [PubMed] [Google Scholar]
- McCormick J. N., Wojtacha D., Edmond E. Detection of cytomegalovirus antigens in phagocytosed serum complexes from a patient with rheumatoid arthritis, vasculitis, peripheral neuropathy, cutaneous ulceration, and digital gangrene. Ann Rheum Dis. 1992 Apr;51(4):553–555. doi: 10.1136/ard.51.4.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelson-Fiske S. Human cytomegalovirus. A review of developments between 1970 and 1976. I. Clinical developments. Biomedicine. 1977 Feb;26(1):16–22. [PubMed] [Google Scholar]
- Munthe E., Natvig J. B. Complement-fixing intracellular complexes of IgG rheumatoid factor in rheumatoid plasma cells. Scand J Immunol. 1972;1(3):217–229. doi: 10.1111/j.1365-3083.1972.tb01813.x. [DOI] [PubMed] [Google Scholar]
- Murayama T., Jisaki F., Ayata M., Sakamuro D., Hironaka T., Hirai K., Tsuchiya N., Ito K., Furukawa T. Cytomegalovirus genomes demonstrated by polymerase chain reaction in synovial fluid from rheumatoid arthritis patients. Clin Exp Rheumatol. 1992 Mar-Apr;10(2):161–164. [PubMed] [Google Scholar]
- Musiani M., Zerbini M., Ferri S., Plazzi M., Gentilomi G., La Placa M. Comparison of the immune response to Epstein-Barr virus and cytomegalovirus in sera and synovial fluids of patients with rheumatoid arthritis. Ann Rheum Dis. 1987 Nov;46(11):837–842. doi: 10.1136/ard.46.11.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemazee D. A., Sato V. L. Enhancing antibody: a novel component of the immune response. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3828–3832. doi: 10.1073/pnas.79.12.3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newkirk M. M., Gram H., Heinrich G. F., Ostberg L., Capra J. D., Wasserman R. L. Complete protein sequences of the variable regions of the cloned heavy and light chains of a human anti-cytomegalovirus antibody reveal a striking similarity to human monoclonal rheumatoid factors of the Wa idiotypic family. J Clin Invest. 1988 May;81(5):1511–1518. doi: 10.1172/JCI113483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newkirk M. M., Mageed R. A., Jefferis R., Chen P. P., Capra J. D. Complete amino acid sequences of variable regions of two human IgM rheumatoid factors, BOR and KAS of the Wa idiotypic family, reveal restricted use of heavy and light chain variable and joining region gene segments. J Exp Med. 1987 Aug 1;166(2):550–564. doi: 10.1084/jem.166.2.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen N. J., Jasin H. E. Decreased pokeweed mitogen-induced IgM and IgM rheumatoid factor synthesis in rheumatoid arthritis patients treated with gold sodium thiomalate or penicillamine. Arthritis Rheum. 1984 Sep;27(9):985–994. doi: 10.1002/art.1780270904. [DOI] [PubMed] [Google Scholar]
- Panayi G. S., Wooley P., Batchelor J. R. Genetic basis of rheumatoid disease: HLA antigens, disease manifestations, and toxic reactions to drugs. Br Med J. 1978 Nov 11;2(6148):1326–1328. doi: 10.1136/bmj.2.6148.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poljak R. J. Crystallization of immunoglobulins and their fragments for X-ray diffraction studies. Methods Enzymol. 1985;116:190–200. doi: 10.1016/s0076-6879(85)16013-1. [DOI] [PubMed] [Google Scholar]
- STERN H., ELEK S. D. THE INCIDENCE OF INFECTION WITH CYTOMEGALOVIRUS IN A NORMAL POPULATION. A SEROLOGICAL STUDY IN GREATER LONDON. J Hyg (Lond) 1965 Mar;63:79–87. doi: 10.1017/s0022172400044983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaughter L., Carson D. A., Jensen F. C., Holbrook T. L., Vaughan J. H. In vitro effects of Epstein-Barr virus on peripheral blood mononuclear cells from patients with rheumatoid arthritis and normal subjects. J Exp Med. 1978 Nov 1;148(5):1429–1434. doi: 10.1084/jem.148.5.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley J. D., Sachs C., Ziff M. In vitro synthesis of immunoglobulin by rheumatoid synovial membrane. J Clin Invest. 1968 Mar;47(3):624–632. doi: 10.1172/JCI105758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagno S., Tinker M. K., Elrod C., Fuccillo D. A., Cloud G., O'Beirne A. J. Immunoglobulin M antibodies detected by enzyme-linked immunosorbent assay and radioimmunoassay in the diagnosis of cytomegalovirus infections in pregnant women and newborn infants. J Clin Microbiol. 1985 Jun;21(6):930–935. doi: 10.1128/jcm.21.6.930-935.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus S. E., Ostrove J. M., Inchauspé G., Felser J. M., Freifeld A., Croen K. D., Sawyer M. H. NIH conference. Varicella-zoster virus infections. Biology, natural history, treatment, and prevention. Ann Intern Med. 1988 Feb;108(2):221–237. doi: 10.7326/0003-4819-108-2-221. [DOI] [PubMed] [Google Scholar]
- Thiele D. L., Kurosaka M., Lipsky P. E. Phenotype of the accessory cell necessary for mitogen-stimulated T and B cell responses in human peripheral blood: delineation by its sensitivity to the lysosomotropic agent, L-leucine methyl ester. J Immunol. 1983 Nov;131(5):2282–2290. [PubMed] [Google Scholar]
- Venables P. J., Ross M. G., Charles P. J., Melsom R. D., Griffiths P. D., Maini R. N. A seroepidemiological study of cytomegalovirus and Epstein-Barr virus in rheumatoid arthritis and sicca syndrome. Ann Rheum Dis. 1985 Nov;44(11):742–746. doi: 10.1136/ard.44.11.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winchester R. J., Agnello V., Kunkel H. G. Gamma globulin complexes in synovial fluids of patients with rheumatoid arthritis. Partial characterization and relationship to lowered complement levels. Clin Exp Immunol. 1970 May;6(5):689–706. [PMC free article] [PubMed] [Google Scholar]



