Abstract
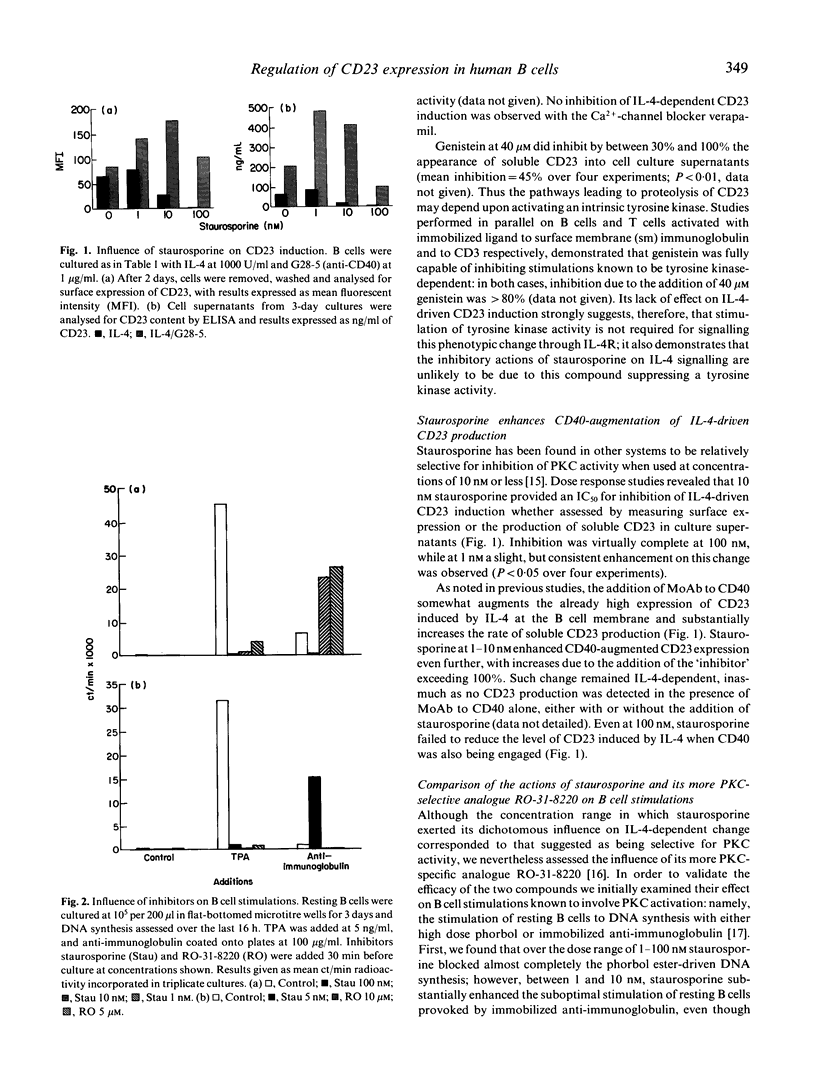
IL-4 synergizes with signals delivered through CD40 both for the induction of CD23/Fc epsilon RII expression and for IgE synthesis. Moreover, engagement of CD40 on the B cell surface by MoAb overcomes the ability of interferons, transforming growth factor-beta, or anti-CD19 to inhibit IL-4-dependent change. We now report that occupancy of CD40 relieves potent suppression of IL-4-induced CD23 production by glucocorticoid or the relatively broad-acting kinase inhibitor staurosporine. Interruption of the IL-4 signal was observed with concentrations of staurosporine considered to be selective for protein kinase C (PKC) inhibition (IC50 = 10 nM) but not with genistein or tyrphostins, effective inhibitors of tyrosine kinase activity. On ligation of CD40, staurosporine no longer inhibited the IL-4 signal: at concentrations of between 1 and 20 nM, staurosporine actually increased by as much as 100% the rate of CD23 production stimulated on simultaneous activation through CD40 and IL-4R. Such augmentation was not observed when the more specific PKC inhibitor RO-31-8220 was used; indeed, CD40 engagement was unable to overcome the ability of this inhibitor to block IL-4-promoted CD23 induction (IC50 = 10 microM). Occupancy of CD40 did, however, thwart completely the usual ability of prednisolone to inhibit the IL-4 signal leading to CD23 induction. Activation through CD40 left inhibition of phorbol ester-induced CD23 expression by staurosporine, RO-31-8220, or glucocorticoid unchecked. These findings further highlight the intimate level of cross-talk existing between CD40 and IL-4R on resting B lymphocytes to promote CD23 expression, a phenotypic change which preludes IgE synthesis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armitage R. J., Fanslow W. C., Strockbine L., Sato T. A., Clifford K. N., Macduff B. M., Anderson D. M., Gimpel S. D., Davis-Smith T., Maliszewski C. R. Molecular and biological characterization of a murine ligand for CD40. Nature. 1992 May 7;357(6373):80–82. doi: 10.1038/357080a0. [DOI] [PubMed] [Google Scholar]
- Banchereau J., de Paoli P., Vallé A., Garcia E., Rousset F. Long-term human B cell lines dependent on interleukin-4 and antibody to CD40. Science. 1991 Jan 4;251(4989):70–72. doi: 10.1126/science.1702555. [DOI] [PubMed] [Google Scholar]
- Cairns J., Flores-Romo L., Millsum M. J., Guy G. R., Gillis S., Ledbetter J. A., Gordon J. Soluble CD23 is released by B lymphocytes cycling in response to interleukin 4 and anti-Bp50 (CDw40). Eur J Immunol. 1988 Mar;18(3):349–353. doi: 10.1002/eji.1830180305. [DOI] [PubMed] [Google Scholar]
- Clark E. A., Ledbetter J. A. Activation of human B cells mediated through two distinct cell surface differentiation antigens, Bp35 and Bp50. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4494–4498. doi: 10.1073/pnas.83.12.4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis P. D., Hill C. H., Keech E., Lawton G., Nixon J. S., Sedgwick A. D., Wadsworth J., Westmacott D., Wilkinson S. E. Potent selective inhibitors of protein kinase C. FEBS Lett. 1989 Dec 18;259(1):61–63. doi: 10.1016/0014-5793(89)81494-2. [DOI] [PubMed] [Google Scholar]
- Delespesse G., Suter U., Mossalayi D., Bettler B., Sarfati M., Hofstetter H., Kilcherr E., Debre P., Dalloul A. Expression, structure, and function of the CD23 antigen. Adv Immunol. 1991;49:149–191. doi: 10.1016/s0065-2776(08)60776-2. [DOI] [PubMed] [Google Scholar]
- Finney M., Guy G. R., Michell R. H., Gordon J., Dugas B., Rigley K. P., Callard R. E. Interleukin 4 activates human B lymphocytes via transient inositol lipid hydrolysis and delayed cyclic adenosine monophosphate generation. Eur J Immunol. 1990 Jan;20(1):151–156. doi: 10.1002/eji.1830200122. [DOI] [PubMed] [Google Scholar]
- Finney M., Michell R. H., Gillis S., Gordon J. Regulation of the interleukin 4 signal in human B-lymphocytes. Biochem Soc Trans. 1991 Apr;19(2):287–291. doi: 10.1042/bst0190287. [DOI] [PubMed] [Google Scholar]
- Gordon J. CD23 and B cell activation. Clin Exp Allergy. 1992 Feb;22(2):199–204. doi: 10.1111/j.1365-2222.1992.tb03073.x. [DOI] [PubMed] [Google Scholar]
- Gordon J., Flores-Romo L., Cairns J. A., Millsum M. J., Lane P. J., Johnson G. D., MacLennan I. C. CD23: a multi-functional receptor/lymphokine? Immunol Today. 1989 May;10(5):153–157. doi: 10.1016/0167-5699(89)90171-0. [DOI] [PubMed] [Google Scholar]
- Gordon J., Katira A., Strain A. J., Gillis S. Inhibition of interleukin 4-promoted CD23 production in human B lymphocytes by transforming growth factor-beta, interferons or anti-CD19 antibody is overriden on engaging CD40. Eur J Immunol. 1991 Aug;21(8):1917–1922. doi: 10.1002/eji.1830210821. [DOI] [PubMed] [Google Scholar]
- Guy G. R., Gordon J. Epstein-Barr virus and a tumour-promoting phorbol ester use similar mechanisms in the stimulation of human B-cell proliferation. Int J Cancer. 1989 Apr 15;43(4):703–708. doi: 10.1002/ijc.2910430427. [DOI] [PubMed] [Google Scholar]
- Guy G. R., Gordon J., Walker L., Michell R. H., Brown G. Redistribution of protein kinase C during mitogenesis of human B lymphocytes. Biochem Biophys Res Commun. 1986 Feb 26;135(1):146–153. doi: 10.1016/0006-291x(86)90954-x. [DOI] [PubMed] [Google Scholar]
- Karin M. Signal transduction and gene control. Curr Opin Cell Biol. 1991 Jun;3(3):467–473. doi: 10.1016/0955-0674(91)90075-a. [DOI] [PubMed] [Google Scholar]
- Tamaoki T., Nomoto H., Takahashi I., Kato Y., Morimoto M., Tomita F. Staurosporine, a potent inhibitor of phospholipid/Ca++dependent protein kinase. Biochem Biophys Res Commun. 1986 Mar 13;135(2):397–402. doi: 10.1016/0006-291x(86)90008-2. [DOI] [PubMed] [Google Scholar]
- Uckun F. M., Schieven G. L., Dibirdik I., Chandan-Langlie M., Tuel-Ahlgren L., Ledbetter J. A. Stimulation of protein tyrosine phosphorylation, phosphoinositide turnover, and multiple previously unidentified serine/threonine-specific protein kinases by the Pan-B-cell receptor CD40/Bp50 at discrete developmental stages of human B-cell ontogeny. J Biol Chem. 1991 Sep 15;266(26):17478–17485. [PubMed] [Google Scholar]
- Wu C. Y., Sarfati M., Heusser C., Fournier S., Rubio-Trujillo M., Peleman R., Delespesse G. Glucocorticoids increase the synthesis of immunoglobulin E by interleukin 4-stimulated human lymphocytes. J Clin Invest. 1991 Mar;87(3):870–877. doi: 10.1172/JCI115092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K., Clark E. A., Saxon A. CD40 stimulation provides an IFN-gamma-independent and IL-4-dependent differentiation signal directly to human B cells for IgE production. J Immunol. 1991 Mar 15;146(6):1836–1842. [PubMed] [Google Scholar]
- de Vries J. E., Gauchat J. F., Aversa G. G., Punnonen J., Gascan H., Yssel H. Regulation of IgE synthesis by cytokines. Curr Opin Immunol. 1991 Dec;3(6):851–858. doi: 10.1016/s0952-7915(05)80003-2. [DOI] [PubMed] [Google Scholar]


