Abstract
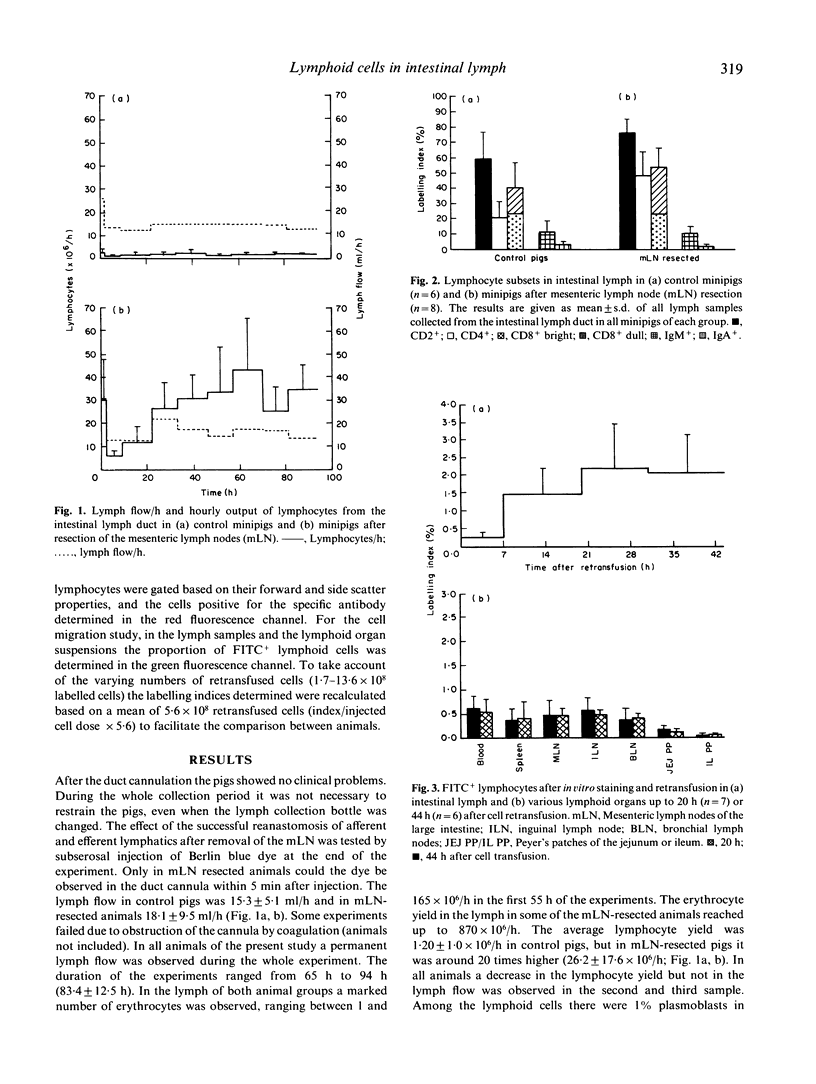
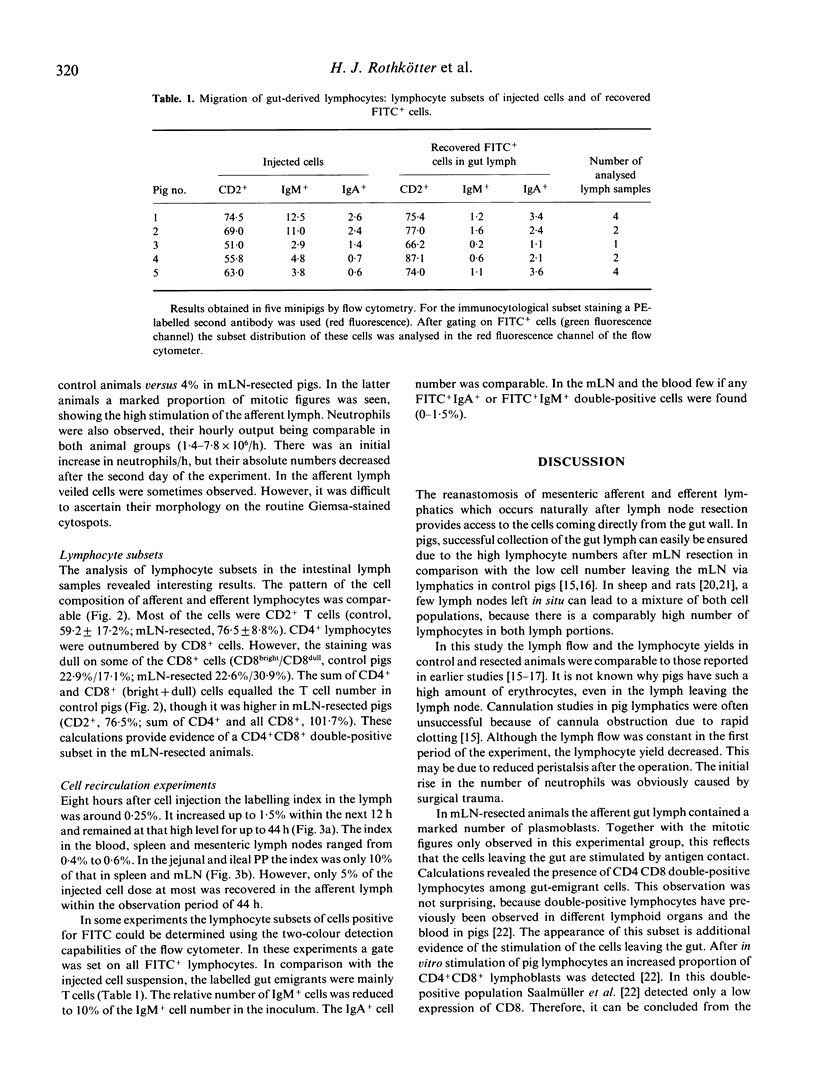
Gut wall emigrating cells have been characterized in the intestinal lymph. The intestinal lymph duct was cannulated in 6-month-old minipigs. Under non-restraining conditions the efferent lymph from the mesenteric lymph nodes was collected in seven normal animals. Lymph coming directly from the gut (afferent lymph) was also collected in 18 pigs after resection of the mesenteric lymph node chains 3 months previously. The intestinal lymph flow was similar in both groups (around 18 ml/h). The lymphoid cell yield was 1.2 +/- 1.0 x 10(6)/h in control animals, while in mesenteric lymph node resected pigs it was around 20 times higher (26.2 +/- 17.6 x 10(6)/h). In the gut-derived lymph 76.5 +/- 8.8% T lymphocytes were observed (CD4+, 48.1 +/- 15.5%; CD8+, 53.6 +/- 12.7%). The percentage of immunoglobulin-positive cells was lower (IgM+, 10.1 +/- 4.5; IgA+, 1.7 +/- 1.1). In 14 mesenteric lymph node resected pigs a mean of 5.6 +/- 3.1 x 10(8) lymphocytes from the gut lymph were labelled in vitro with a fluorescent dye and retransfused. The labelling index of fluorescent cells in the intestinal lymph increased rapidly and remained at a high level until 44 h after cell transfusion. A four-to-ten times lower labelling index was found in the spleen, various lymph nodes and Peyer's patches. Most of the recovered lymphocytes were T cells. This model provides access to the cell pool leaving the gut wall, thus allowing an examination of its role in the gastrointestinal tract and other mucosal-lined organs.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennell M. A., Husband A. J. Route of lymphocyte migration in pigs. I. Lymphocyte circulation in gut-associated lymphoid tissue. Immunology. 1981 Mar;42(3):469–474. [PMC free article] [PubMed] [Google Scholar]
- Bennell M. A., Watson D. L. Immunological parameters in the intestinal lymph of pigs including changes during experimentally induced diarrhoea. Res Vet Sci. 1979 May;26(3):284–288. [PubMed] [Google Scholar]
- Bienenstock J., Befus D., McDermott M., Mirski S., Rosenthal K. Regulation of lymphoblast traffic and localization in mucosal tissues, with emphasis on IgA. Fed Proc. 1983 Dec;42(15):3213–3217. [PubMed] [Google Scholar]
- Binns R. M., Blakeley D., Licence S. T. Migration of fluoresceinated pig lymphocytes in vivo: technical aspects and use in studies of autologous and homologous cell survival for up to three weeks. Int Arch Allergy Appl Immunol. 1981;66(3):341–349. doi: 10.1159/000232839. [DOI] [PubMed] [Google Scholar]
- Binns R. M., Licence S. T. Patterns of migration of labelled blood lymphocyte subpopulations: evidence for two types of Peyer's patch in the young pig. Adv Exp Med Biol. 1985;186:661–668. doi: 10.1007/978-1-4613-2463-8_81. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P., Halstensen T. S., Kett K., Krajci P., Kvale D., Rognum T. O., Scott H., Sollid L. M. Immunobiology and immunopathology of human gut mucosa: humoral immunity and intraepithelial lymphocytes. Gastroenterology. 1989 Dec;97(6):1562–1584. doi: 10.1016/0016-5085(89)90406-x. [DOI] [PubMed] [Google Scholar]
- Delventhal S., Hensel A., Petzoldt K., Pabst R. Cellular changes in the bronchoalveolar lavage (BAL) of pigs, following immunization by the enteral or respiratory route. Clin Exp Immunol. 1992 Nov;90(2):223–227. doi: 10.1111/j.1365-2249.1992.tb07933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkley M. L., Husband A. J. Role of antigen in migration patterns of T cell subsets arising from gut-associated lymphoid tissue. Reg Immunol. 1989 Jul-Aug;2(4):213–224. [PubMed] [Google Scholar]
- Dunkley M. L., Husband A. J. The role of non-B cells in localizing an IgA plasma cell response in the intestine. Reg Immunol. 1990;3(6):336–340. [PubMed] [Google Scholar]
- Gerber H. A., Morris B., Trevella W. The role of gut-associated lymphoid tissues in the generation of immunoglobulin-bearing lymphocytes in sheep. Aust J Exp Biol Med Sci. 1986 Jun;64(Pt 3):201–213. doi: 10.1038/icb.1986.22. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Ramos J. C., Pezzi L., Palacios R., Martínez C. Expression of the p75 interleukin 2-binding protein on CD3+4-8-Tac- cells from autoimmune MRL/MP-lpr/lpr mice. Eur J Immunol. 1989 Jan;19(1):201–204. doi: 10.1002/eji.1830190133. [DOI] [PubMed] [Google Scholar]
- Guy-Grand D., Griscelli C., Vassalli P. The gut-associated lymphoid system: nature and properties of the large dividing cells. Eur J Immunol. 1974 Jun;4(6):435–443. doi: 10.1002/eji.1830040610. [DOI] [PubMed] [Google Scholar]
- Jalkanen S., Nash G. S., De los Toyos J., MacDermott R. P., Butcher E. C. Human lamina propria lymphocytes bear homing receptors and bind selectively to mucosal lymphoid high endothelium. Eur J Immunol. 1989 Jan;19(1):63–68. doi: 10.1002/eji.1830190111. [DOI] [PubMed] [Google Scholar]
- Jeurissen S. H., Duijvestijn A. M., Sontag Y., Kraal G. Lymphocyte migration into the lamina propria of the gut is mediated by specialized HEV-like blood vessels. Immunology. 1987 Oct;62(2):273–277. [PMC free article] [PubMed] [Google Scholar]
- Jeurissen S. H., Sminia T., Kraal G. Selective emigration of suppressor T cells from Peyer's patches. Cell Immunol. 1984 Apr 15;85(1):264–269. doi: 10.1016/0008-8749(84)90297-1. [DOI] [PubMed] [Google Scholar]
- MANN J. D., HIGGINS G. M. Lymphocytes in thoracic duct intestinal and hepatic lymph. Blood. 1950 Feb;5(2):177–190. [PubMed] [Google Scholar]
- Mackay C. R., Marston W. L., Dudler L., Spertini O., Tedder T. F., Hein W. R. Tissue-specific migration pathways by phenotypically distinct subpopulations of memory T cells. Eur J Immunol. 1992 Apr;22(4):887–895. doi: 10.1002/eji.1830220402. [DOI] [PubMed] [Google Scholar]
- Pabst R., Geist M., Rothkötter H. J., Fritz F. J. Postnatal development and lymphocyte production of jejunal and ileal Peyer's patches in normal and gnotobiotic pigs. Immunology. 1988 Jul;64(3):539–544. [PMC free article] [PubMed] [Google Scholar]
- Pabst R., Reynolds J. D. Peyer's patches export lymphocytes throughout the lymphoid system in sheep. J Immunol. 1987 Dec 15;139(12):3981–3985. [PubMed] [Google Scholar]
- Pabst R. The anatomical basis for the immune function of the gut. Anat Embryol (Berl) 1987;176(2):135–144. doi: 10.1007/BF00310046. [DOI] [PubMed] [Google Scholar]
- Reynolds J. D., Pabst R. The emigration of lymphocytes from Peyer's patches in sheep. Eur J Immunol. 1984 Jan;14(1):7–13. doi: 10.1002/eji.1830140103. [DOI] [PubMed] [Google Scholar]
- Rothkötter H. J., Zimmermann H. J., Pabst R. Size of jejunal Peyer's patches and migration of lymphocyte subsets in pigs after resection or transposition of the continuous ileal Peyer's patch. Scand J Immunol. 1990 Feb;31(2):191–197. doi: 10.1111/j.1365-3083.1990.tb02759.x. [DOI] [PubMed] [Google Scholar]
- Saalmüller A., Reddehase M. J., Bühring H. J., Jonjić S., Koszinowski U. H. Simultaneous expression of CD4 and CD8 antigens by a substantial proportion of resting porcine T lymphocytes. Eur J Immunol. 1987 Sep;17(9):1297–1301. doi: 10.1002/eji.1830170912. [DOI] [PubMed] [Google Scholar]
- Weisz-Carrington P., Emancipator S., Kelemen P. R. Specific attachment of mesenteric IgA lymphoblasts to specialized endothelium of intestinal mucosa lamina propria capillaries. Cell Immunol. 1991 Feb;132(2):494–504. doi: 10.1016/0008-8749(91)90045-d. [DOI] [PubMed] [Google Scholar]


