Abstract
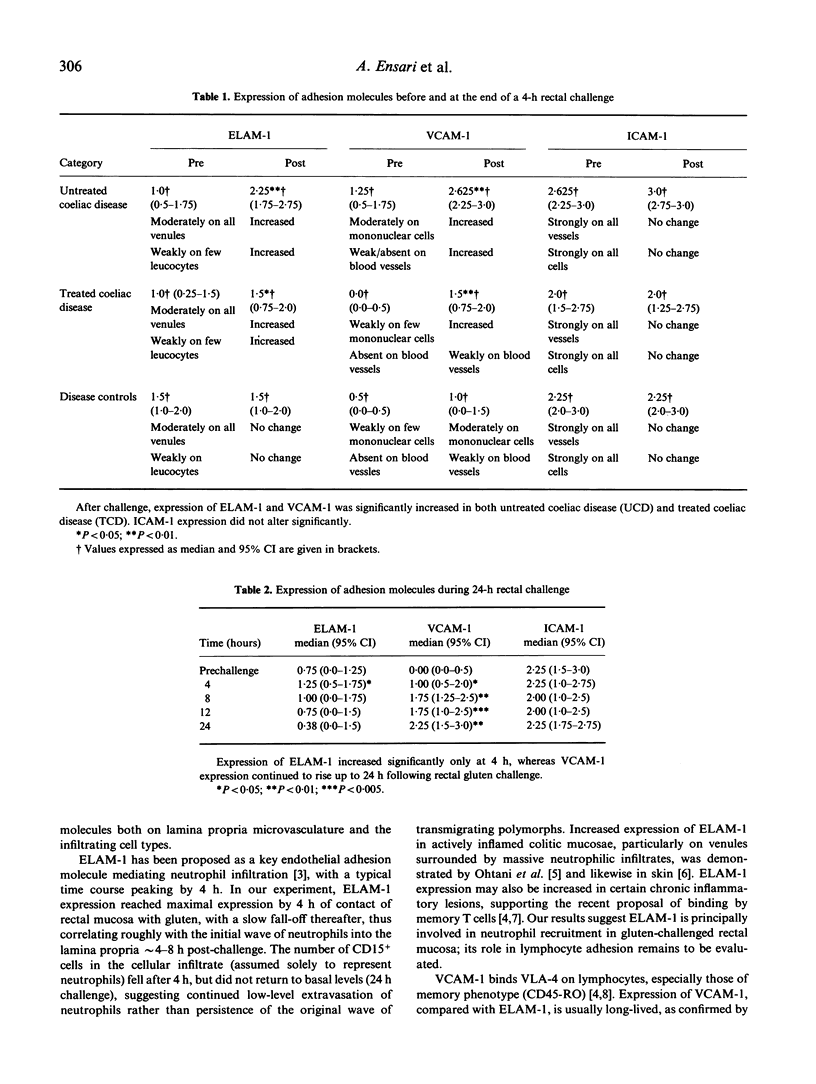
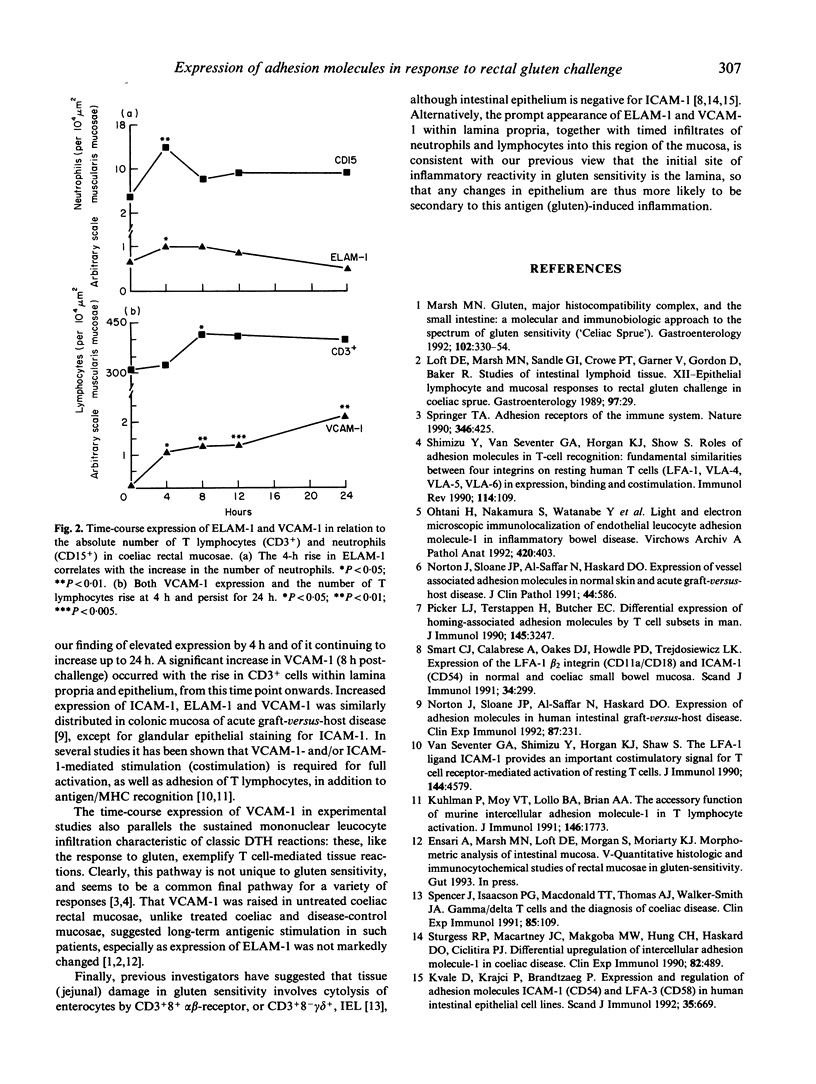
Adhesive interactions between endothelium and circulating cells, such as monocytes, neutrophils and lymphocytes, are crucial for localizing the inflammatory response. We investigated the inflammatory response of rectal mucosa to local gluten challenge as a dynamic model of antigen-induced tissue injury, during which the expression of adhesion molecules on leucocytes and endothelial cells could be sequentially observed. Expression of ELAM-1, ICAM-1 and VCAM-1 was monitored in 10 treated and eight untreated patients with gluten sensitivity (coeliac disease), and in five disease controls for up to 4 h (short challenge), while a further seven treated coeliacs were monitored for up to 24 h (long challenge) following rectal gluten challenge. In the former, the expression of VCAM-1 and ELAM-1 was significantly raised 4 h after gluten challenge compared with controls. VCAM-1 and ELAM-1 expression was also increased in mucosae of treated patients, but to a lesser extent. VCAM-1 expression continued to increase for up to 24 h after gluten, while ELAM-1 had begun to wane by 4 h, reaching basal levels by 24 h. In contrast, the expression of ICAM-1 did not change in any of the disease groups studied. These findings relate to significant increases in lymphocytes (CD3+ cells) after 8 h, and neutrophils (CD15+ cells) after 4 h in the lamina propria. This approach has permitted novel studies of the inflammatory response to a defined antigen in sensitized (gluten-sensitive) human patients.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Kuhlman P., Moy V. T., Lollo B. A., Brian A. A. The accessory function of murine intercellular adhesion molecule-1 in T lymphocyte activation. Contributions of adhesion and co-activation. J Immunol. 1991 Mar 15;146(6):1773–1782. [PubMed] [Google Scholar]
- Kvale D., Krajci P., Brandtzaeg P. Expression and regulation of adhesion molecules ICAM-1 (CD54) and LFA-3 (CD58) in human intestinal epithelial cell lines. Scand J Immunol. 1992 Jun;35(6):669–676. doi: 10.1111/j.1365-3083.1992.tb02973.x. [DOI] [PubMed] [Google Scholar]
- Loft D. E., Marsh M. N., Sandle G. I., Crowe P. T., Garner V., Gordon D., Baker R. Studies of intestinal lymphoid tissue. XII. Epithelial lymphocyte and mucosal responses to rectal gluten challenge in celiac sprue. Gastroenterology. 1989 Jul;97(1):29–37. doi: 10.1016/0016-5085(89)91411-x. [DOI] [PubMed] [Google Scholar]
- Marsh M. N. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity ('celiac sprue'). Gastroenterology. 1992 Jan;102(1):330–354. [PubMed] [Google Scholar]
- Norton J., Sloane J. P., al-Saffar N., Haskard D. O. Expression of adhesion molecules in human intestinal graft-versus-host disease. Clin Exp Immunol. 1992 Feb;87(2):231–236. doi: 10.1111/j.1365-2249.1992.tb02980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton J., Sloane J. P., al-Saffar N., Haskard D. O. Vessel associated adhesion molecules in normal skin and acute graft-versus-host disease. J Clin Pathol. 1991 Jul;44(7):586–591. doi: 10.1136/jcp.44.7.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtani H., Nakamura S., Watanabe Y., Fukushima K., Mizoi T., Kimura M., Hiwatashi N., Nagura H. Light and electron microscopic immunolocalization of endothelial leucocyte adhesion molecule-1 in inflammatory bowel disease. Morphological evidence of active synthesis and secretion into vascular lumen. Virchows Arch A Pathol Anat Histopathol. 1992;420(5):403–409. doi: 10.1007/BF01600511. [DOI] [PubMed] [Google Scholar]
- Picker L. J., Terstappen L. W., Rott L. S., Streeter P. R., Stein H., Butcher E. C. Differential expression of homing-associated adhesion molecules by T cell subsets in man. J Immunol. 1990 Nov 15;145(10):3247–3255. [PubMed] [Google Scholar]
- Shimizu Y., van Seventer G. A., Horgan K. J., Shaw S. Roles of adhesion molecules in T-cell recognition: fundamental similarities between four integrins on resting human T cells (LFA-1, VLA-4, VLA-5, VLA-6) in expression, binding, and costimulation. Immunol Rev. 1990 Apr;114:109–143. doi: 10.1111/j.1600-065x.1990.tb00563.x. [DOI] [PubMed] [Google Scholar]
- Smart C. J., Calabrese A., Oakes D. J., Howdle P. D., Trejdosiewicz L. K. Expression of the LFA-1 beta 2 integrin (CD11a/CD18) and ICAM-1 (CD54) in normal and coeliac small bowel mucosa. Scand J Immunol. 1991 Sep;34(3):299–305. doi: 10.1111/j.1365-3083.1991.tb01550.x. [DOI] [PubMed] [Google Scholar]
- Spencer J., Isaacson P. G., MacDonald T. T., Thomas A. J., Walker-Smith J. A. Gamma/delta T cells and the diagnosis of coeliac disease. Clin Exp Immunol. 1991 Jul;85(1):109–113. doi: 10.1111/j.1365-2249.1991.tb05690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Sturgess R. P., Macartney J. C., Makgoba M. W., Hung C. H., Haskard D. O., Ciclitira P. J. Differential upregulation of intercellular adhesion molecule-1 in coeliac disease. Clin Exp Immunol. 1990 Dec;82(3):489–492. doi: 10.1111/j.1365-2249.1990.tb05477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Seventer G. A., Shimizu Y., Horgan K. J., Shaw S. The LFA-1 ligand ICAM-1 provides an important costimulatory signal for T cell receptor-mediated activation of resting T cells. J Immunol. 1990 Jun 15;144(12):4579–4586. [PubMed] [Google Scholar]