Abstract
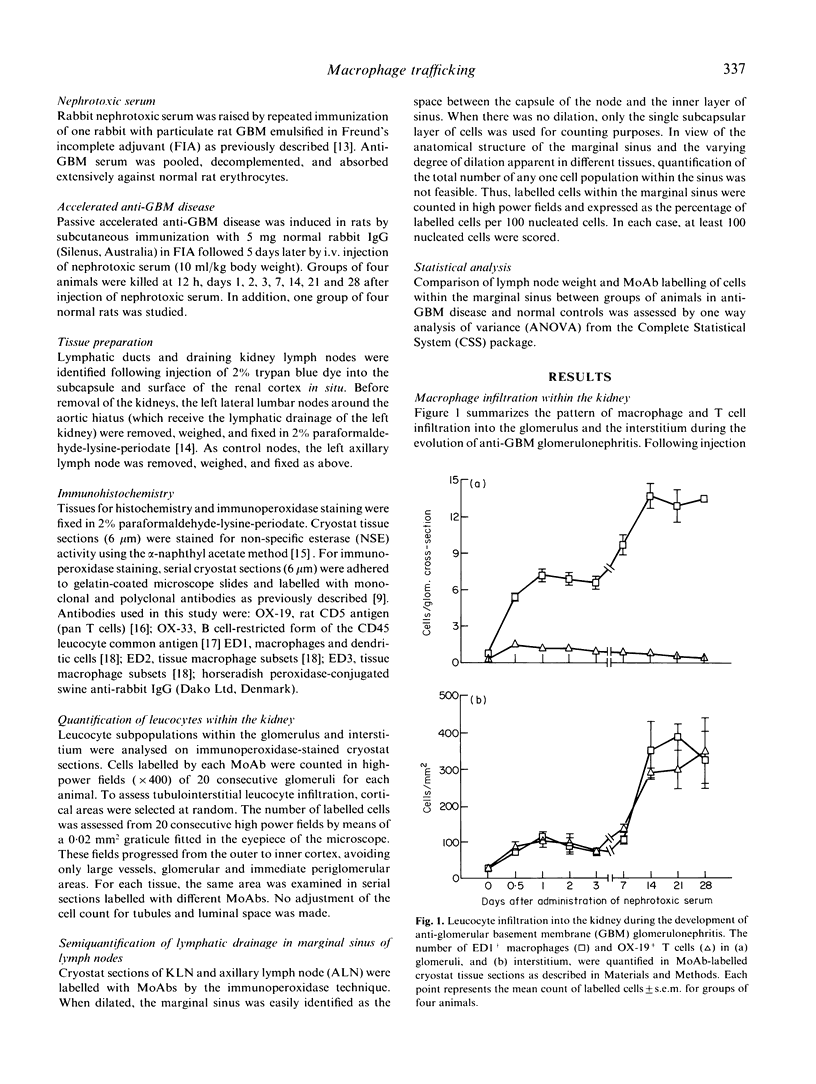
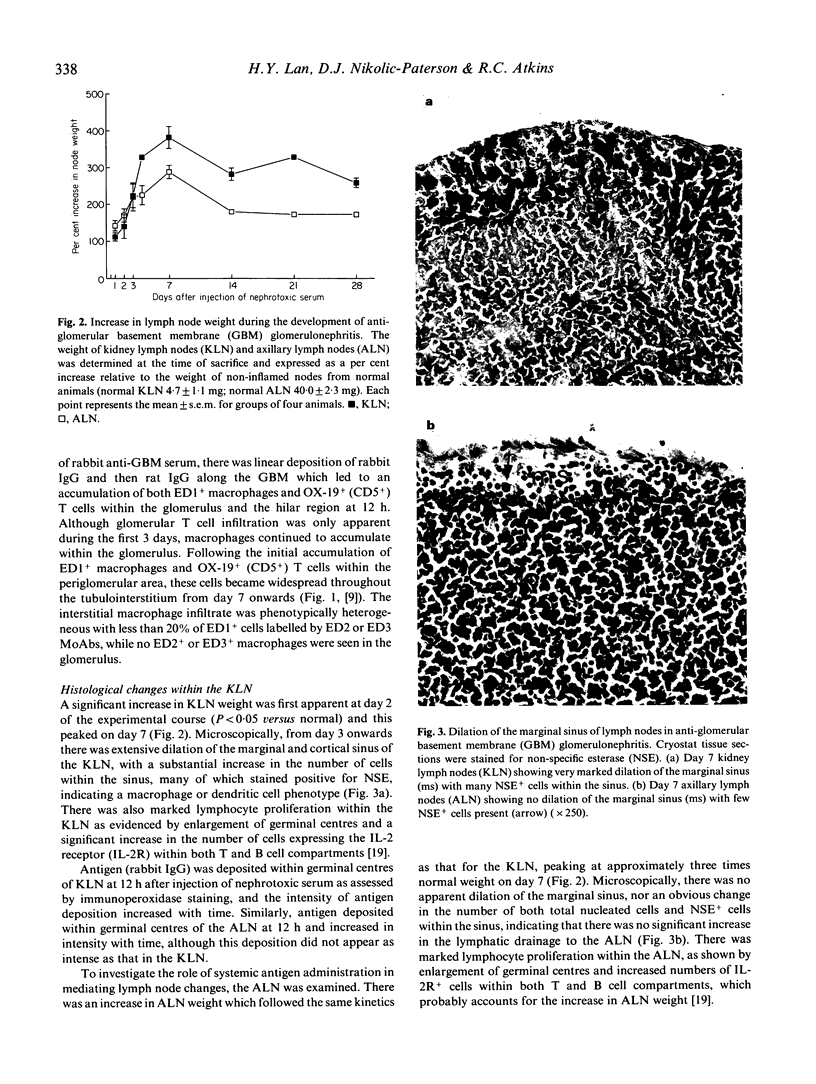
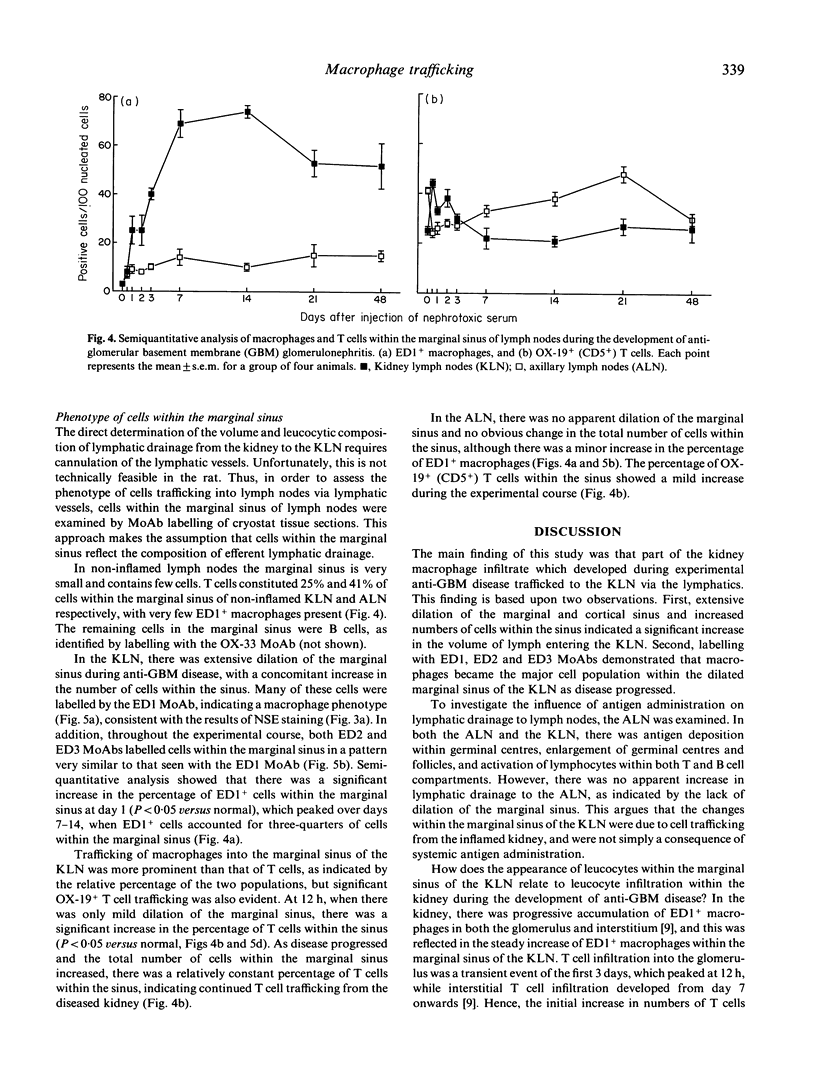
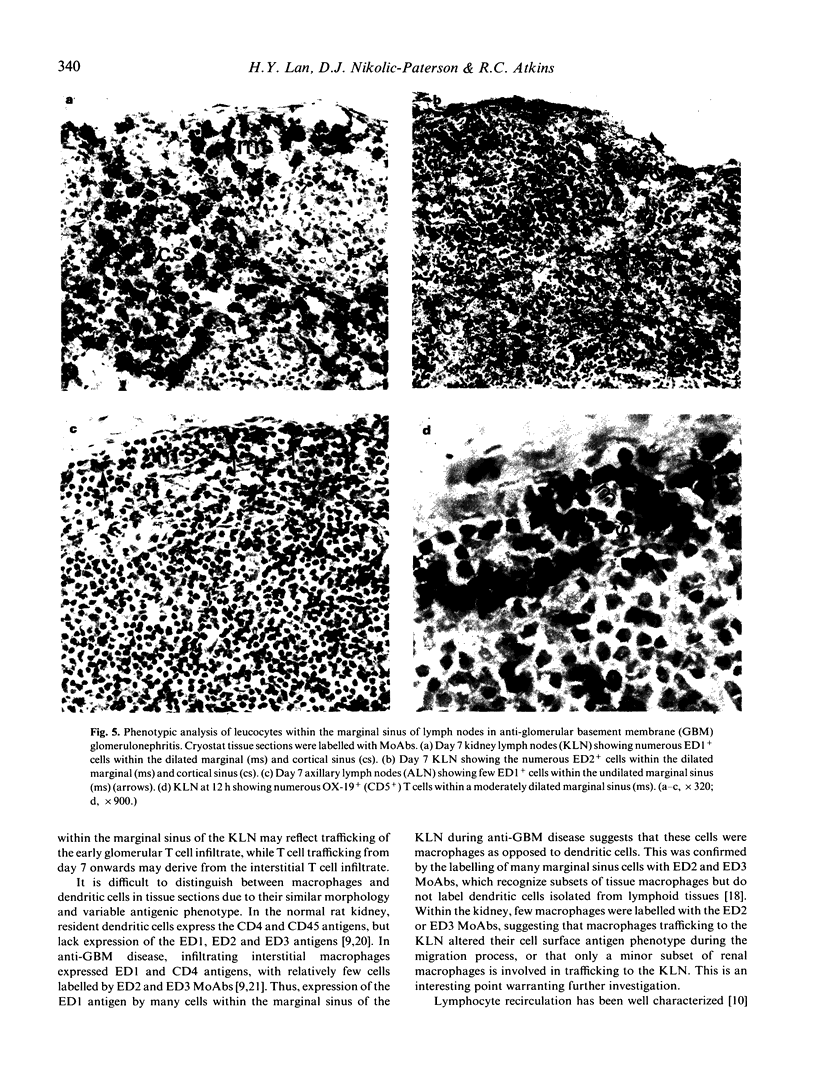
Macrophage accumulation within the glomerulus and renal interstitium is a prominent feature of most forms of glomerulonephritis, but the fate of these inflammatory cells is unknown. Macrophage trafficking to the draining kidney lymph nodes (KLN) was assessed in a detailed kinetic analysis of accelerated antiglomerular basement membrane (GBM) disease in the rat. Leucocytes draining to KLN via lymphatic vessels were identified within the marginal sinus by MoAb labelling of tissue sections. In anti-GBM disease, there was a significant increase in the weight of the KLN due to both lymphoproliferation within the nodes and increased lymphatic drainage from the inflamed kidney, as evidenced by prominent dilation of the marginal sinus and increased numbers of cells within the sinus. In non-inflamed lymph nodes, few ED1+ macrophages were present within the marginal sinus (3.0 +/- 0.6/100 nucleated cells). However, in anti-GBM disease, macrophages became the major cell type within the dilated marginal sinus of the KLN, as shown by labelling with ED1, ED2 and ED3 MoAbs, peaking at 74 +/- 2.6 ED1+ cells/100 nucleated cells at day 14. These changes were not simply due to systemic antigen administration, since in the axillary lymph node (ALN) there was no obvious dilation of the marginal sinus and macrophages accounted for a maximum of only 15 +/- 4.6 ED1+ cells/100 nucleated cells. In conclusion, this study provides indirect evidence that there is significant trafficking of the renal macrophage infiltrate to the KLN during experimental glomerulonephritis. This may be a mechanism whereby nephritogenic antigens, released as a consequence of the local inflammatory response, may be presented to T and B lymphocytes within lymph nodes, resulting in the amplification of the immune response in glomerulonephritis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexopoulos E., Seron D., Hartley R. B., Cameron J. S. Lupus nephritis: correlation of interstitial cells with glomerular function. Kidney Int. 1990 Jan;37(1):100–109. doi: 10.1038/ki.1990.14. [DOI] [PubMed] [Google Scholar]
- Cumberbatch M., Kimber I. Phenotypic characteristics of antigen-bearing cells in the draining lymph nodes of contact sensitized mice. Immunology. 1990 Nov;71(3):404–410. [PMC free article] [PubMed] [Google Scholar]
- Dallman M. J., Thomas M. L., Green J. R. MRC OX-19: a monoclonal antibody that labels rat T lymphocytes and augments in vitro proliferative responses. Eur J Immunol. 1984 Mar;14(3):260–267. doi: 10.1002/eji.1830140311. [DOI] [PubMed] [Google Scholar]
- Dijkstra C. D., Döpp E. A., Joling P., Kraal G. The heterogeneity of mononuclear phagocytes in lymphoid organs: distinct macrophage subpopulations in the rat recognized by monoclonal antibodies ED1, ED2 and ED3. Immunology. 1985 Mar;54(3):589–599. [PMC free article] [PubMed] [Google Scholar]
- Ferrari F. A., Maccario R., Marconi M., Vitiello M. A., Ugazio A. G., Burgio V., Siccardi A. G. Reliability of alpha-naphthyl-acetate esterase staining of blood smears for the enumeration of circulating human T lymphocytes. Clin Exp Immunol. 1980 Aug;41(2):358–362. [PMC free article] [PubMed] [Google Scholar]
- Gordon S., Crocker P. R., Morris L., Lee S. H., Perry V. H., Hume D. A. Localization and function of tissue macrophages. Ciba Found Symp. 1986;118:54–67. doi: 10.1002/9780470720998.ch5. [DOI] [PubMed] [Google Scholar]
- Hancock W. W., Becker G. J., Atkins R. C. A comparison of fixatives and immunohistochemical technics for use with monoclonal antibodies to cell surface antigens. Am J Clin Pathol. 1982 Dec;78(6):825–831. doi: 10.1093/ajcp/78.6.825. [DOI] [PubMed] [Google Scholar]
- Holdsworth S. R., Neale T. J. Macrophage-induced glomerular injury. Cell transfer studies in passive autologous antiglomerular basement membrane antibody-initiated experimental glomerulonephritis. Lab Invest. 1984 Aug;51(2):172–180. [PubMed] [Google Scholar]
- Holdsworth S. R., Thomson N. M., Glasgow E. F., Dowling J. P., Atkins R. C. Tissue culture of isolated glomeruli in experimental crescentic glomerulonephritis. J Exp Med. 1978 Jan 1;147(1):98–109. doi: 10.1084/jem.147.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooke D. H., Gee D. C., Atkins R. C. Leukocyte analysis using monoclonal antibodies in human glomerulonephritis. Kidney Int. 1987 Apr;31(4):964–972. doi: 10.1038/ki.1987.93. [DOI] [PubMed] [Google Scholar]
- Klahr S., Schreiner G., Ichikawa I. The progression of renal disease. N Engl J Med. 1988 Jun 23;318(25):1657–1666. doi: 10.1056/NEJM198806233182505. [DOI] [PubMed] [Google Scholar]
- Kupiec-Weglinski J. W., Austyn J. M., Morris P. J. Migration patterns of dendritic cells in the mouse. Traffic from the blood, and T cell-dependent and -independent entry to lymphoid tissues. J Exp Med. 1988 Feb 1;167(2):632–645. doi: 10.1084/jem.167.2.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan H. Y., Paterson D. J., Atkins R. C. Initiation and evolution of interstitial leukocytic infiltration in experimental glomerulonephritis. Kidney Int. 1991 Sep;40(3):425–433. doi: 10.1038/ki.1991.229. [DOI] [PubMed] [Google Scholar]
- Li H. L., Hancock W. W., Dowling J. P., Atkins R. C. Activated (IL-2R+) intraglomerular mononuclear cells in crescentic glomerulonephritis. Kidney Int. 1991 Apr;39(4):793–798. doi: 10.1038/ki.1991.97. [DOI] [PubMed] [Google Scholar]
- Main I. W., Nikolic-Paterson D. J., Atkins R. C. T cells and macrophages and their role in renal injury. Semin Nephrol. 1992 Sep;12(5):395–407. [PubMed] [Google Scholar]
- Nolasco F. E., Cameron J. S., Hartley B., Coelho A., Hildreth G., Reuben R. Intraglomerular T cells and monocytes in nephritis: study with monoclonal antibodies. Kidney Int. 1987 May;31(5):1160–1166. doi: 10.1038/ki.1987.123. [DOI] [PubMed] [Google Scholar]
- Pabst R., Binns R. M. Heterogeneity of lymphocyte homing physiology: several mechanisms operate in the control of migration to lymphoid and non-lymphoid organs in vivo. Immunol Rev. 1989 Apr;108:83–109. doi: 10.1111/j.1600-065x.1989.tb00014.x. [DOI] [PubMed] [Google Scholar]
- Sabadini E., Castiglione A., Colasanti G., Ferrario F., Civardi R., Fellin G., D'Amico G. Characterization of interstitial infiltrating cells in Berger's disease. Am J Kidney Dis. 1988 Oct;12(4):307–315. doi: 10.1016/s0272-6386(88)80225-7. [DOI] [PubMed] [Google Scholar]
- Steiniger B., Klempnauer J., Wonigeit K. Phenotype and histological distribution of interstitial dendritic cells in the rat pancreas, liver, heart, and kidney. Transplantation. 1984 Aug;38(2):169–174. doi: 10.1097/00007890-198408000-00016. [DOI] [PubMed] [Google Scholar]
- Szakal A. K., Holmes K. L., Tew J. G. Transport of immune complexes from the subcapsular sinus to lymph node follicles on the surface of nonphagocytic cells, including cells with dendritic morphology. J Immunol. 1983 Oct;131(4):1714–1727. [PubMed] [Google Scholar]
- Woollett G. R., Barclay A. N., Puklavec M., Williams A. F. Molecular and antigenic heterogeneity of the rat leukocyte-common antigen from thymocytes and T and B lymphocytes. Eur J Immunol. 1985 Feb;15(2):168–173. doi: 10.1002/eji.1830150211. [DOI] [PubMed] [Google Scholar]




