Abstract
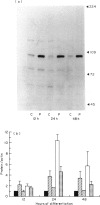
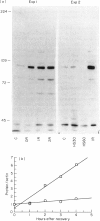
During the phorbol myristate acetate (PMA)-induced differentiation of U937 cells to a macrophage-like phenotype, the levels of the heat shock proteins hsp90, hsp72 and hsp65 increased dramatically to a peak level following 24 h of treatment, and then declined. In contrast, no significant increase was observed in the level of the constitutive hsp73 protein in this process. The observed increases in hsp levels were preceded by an increase in the transcription of each of the genes encoding these hsps, including both of the two genes which encode hsp90. The mechanism of this effect and the possible role of the hsps in the function of differentiated macrophages and in the differentiation process are discussed.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bansal G. S., Norton P. M., Latchman D. S. The 90-kDa heat shock protein protects mammalian cells from thermal stress but not from viral infection. Exp Cell Res. 1991 Aug;195(2):303–306. doi: 10.1016/0014-4827(91)90377-7. [DOI] [PubMed] [Google Scholar]
- Bensaude O., Babinet C., Morange M., Jacob F. Heat shock proteins, first major products of zygotic gene activity in mouse embryo. Nature. 1983 Sep 22;305(5932):331–333. doi: 10.1038/305331a0. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Ellis R. J. The molecular chaperone concept. Semin Cell Biol. 1990 Feb;1(1):1–9. [PubMed] [Google Scholar]
- Finlay C. A., Hinds P. W., Tan T. H., Eliyahu D., Oren M., Levine A. J. Activating mutations for transformation by p53 produce a gene product that forms an hsc70-p53 complex with an altered half-life. Mol Cell Biol. 1988 Feb;8(2):531–539. doi: 10.1128/mcb.8.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granelli-Piperno A., Andrus L., Steinman R. M. Lymphokine and nonlymphokine mRNA levels in stimulated human T cells. Kinetics, mitogen requirements, and effects of cyclosporin A. J Exp Med. 1986 Apr 1;163(4):922–937. doi: 10.1084/jem.163.4.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haire R. N., O'Leary J. J. Mitogen-induced preferential synthesis of proteins during the G0 to S phase transition in human lymphocytes. Exp Cell Res. 1988 Nov;179(1):65–78. doi: 10.1016/0014-4827(88)90349-7. [DOI] [PubMed] [Google Scholar]
- Haire R. N., Peterson M. S., O'Leary J. J. Mitogen activation induces the enhanced synthesis of two heat-shock proteins in human lymphocytes. J Cell Biol. 1988 Mar;106(3):883–891. doi: 10.1083/jcb.106.3.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen L. K., Houchins J. P., O'Leary J. J. Differential regulation of HSC70, HSP70, HSP90 alpha, and HSP90 beta mRNA expression by mitogen activation and heat shock in human lymphocytes. Exp Cell Res. 1991 Feb;192(2):587–596. doi: 10.1016/0014-4827(91)90080-e. [DOI] [PubMed] [Google Scholar]
- Hensold J. O., Hunt C. R., Calderwood S. K., Housman D. E., Kingston R. E. DNA binding of heat shock factor to the heat shock element is insufficient for transcriptional activation in murine erythroleukemia cells. Mol Cell Biol. 1990 Apr;10(4):1600–1608. doi: 10.1128/mcb.10.4.1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey E., Brandon S. E., Sadis S., Smale G., Weber L. A. Molecular cloning of sequences encoding the human heat-shock proteins and their expression during hyperthermia. Gene. 1986;43(1-2):147–154. doi: 10.1016/0378-1119(86)90018-1. [DOI] [PubMed] [Google Scholar]
- Hickey E., Brandon S. E., Smale G., Lloyd D., Weber L. A. Sequence and regulation of a gene encoding a human 89-kilodalton heat shock protein. Mol Cell Biol. 1989 Jun;9(6):2615–2626. doi: 10.1128/mcb.9.6.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard G., Geoghegan T. E. Altered cardiac tissue gene expression during acute hypoxic exposure. Mol Cell Biochem. 1986 Feb;69(2):155–160. doi: 10.1007/BF00224762. [DOI] [PubMed] [Google Scholar]
- Jindal S., Dudani A. K., Singh B., Harley C. B., Gupta R. S. Primary structure of a human mitochondrial protein homologous to the bacterial and plant chaperonins and to the 65-kilodalton mycobacterial antigen. Mol Cell Biol. 1989 May;9(5):2279–2283. doi: 10.1128/mcb.9.5.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H. Heat shock proteins and the immune response. Immunol Today. 1990 Apr;11(4):129–136. doi: 10.1016/0167-5699(90)90050-j. [DOI] [PubMed] [Google Scholar]
- Kemp L. M., Brickell P. M., La Thangue N. B., Latchman D. S. Transcriptional induction of cellular gene expression during lytic infection with herpes simplex virus. Biosci Rep. 1986 Nov;6(11):945–951. doi: 10.1007/BF01114970. [DOI] [PubMed] [Google Scholar]
- Krawczyk Z., Szymik N., Wiśniewski J. Expression of hsp70-related gene in developing and degenerating rat testis. Mol Biol Rep. 1987;12(1):35–41. doi: 10.1007/BF00580648. [DOI] [PubMed] [Google Scholar]
- Latchman D. S. Heat shock proteins and human disease. J R Coll Physicians Lond. 1991 Oct;25(4):295–299. [PMC free article] [PubMed] [Google Scholar]
- Levine R. A., LaRosa G. J., Gudas L. J. Isolation of cDNA clones for genes exhibiting reduced expression after differentiation of murine teratocarcinoma stem cells. Mol Cell Biol. 1984 Oct;4(10):2142–2150. doi: 10.1128/mcb.4.10.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist S., Craig E. A. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Lüscher B., Eisenman R. N. c-myc and c-myb protein degradation: effect of metabolic inhibitors and heat shock. Mol Cell Biol. 1988 Jun;8(6):2504–2512. doi: 10.1128/mcb.8.6.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton P. M., Isenberg D. A., Latchman D. S. Elevated levels of the 90 kd heat shock protein in a proportion of SLE patients with active disease. J Autoimmun. 1989 Apr;2(2):187–195. doi: 10.1016/0896-8411(89)90154-6. [DOI] [PubMed] [Google Scholar]
- Patel R., Chan W. L., Kemp L. M., La Thangue N. B., Latchman D. S. Isolation of cDNA clones derived from a cellular gene transcriptionally induced by herpes simplex virus. Nucleic Acids Res. 1986 Jul 25;14(14):5629–5640. doi: 10.1093/nar/14.14.5629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R. A regulatory upstream promoter element in the Drosophila hsp 70 heat-shock gene. Cell. 1982 Sep;30(2):517–528. doi: 10.1016/0092-8674(82)90249-5. [DOI] [PubMed] [Google Scholar]
- Polla B. S. A role for heat shock proteins in inflammation? Immunol Today. 1988 May;9(5):134–137. doi: 10.1016/0167-5699(88)91199-1. [DOI] [PubMed] [Google Scholar]
- Polla B. S., Healy A. M., Wojno W. C., Krane S. M. Hormone 1 alpha,25-dihydroxyvitamin D3 modulates heat shock response in monocytes. Am J Physiol. 1987 Jun;252(6 Pt 1):C640–C649. doi: 10.1152/ajpcell.1987.252.6.C640. [DOI] [PubMed] [Google Scholar]
- Rebbe N. F., Ware J., Bertina R. M., Modrich P., Stafford D. W. Nucleotide sequence of a cDNA for a member of the human 90-kDa heat-shock protein family. Gene. 1987;53(2-3):235–245. doi: 10.1016/0378-1119(87)90012-6. [DOI] [PubMed] [Google Scholar]
- Richards F. M., Watson A., Hickman J. A. Investigation of the effects of heat shock and agents which induce a heat shock response on the induction of differentiation of HL-60 cells. Cancer Res. 1988 Dec 1;48(23):6715–6720. [PubMed] [Google Scholar]
- Riehl R. M., Sullivan W. P., Vroman B. T., Bauer V. J., Pearson G. R., Toft D. O. Immunological evidence that the nonhormone binding component of avian steroid receptors exists in a wide range of tissues and species. Biochemistry. 1985 Nov 5;24(23):6586–6591. doi: 10.1021/bi00344a042. [DOI] [PubMed] [Google Scholar]
- Sargent C. A., Dunham I., Trowsdale J., Campbell R. D. Human major histocompatibility complex contains genes for the major heat shock protein HSP70. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1968–1972. doi: 10.1073/pnas.86.6.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M. K., Yu J. Accumulation of a heat shock-like protein during differentiation of human erythroid cell line K562. Nature. 1984 Jun 14;309(5969):631–633. doi: 10.1038/309631a0. [DOI] [PubMed] [Google Scholar]
- Sistonen L., Sarge K. D., Phillips B., Abravaya K., Morimoto R. I. Activation of heat shock factor 2 during hemin-induced differentiation of human erythroleukemia cells. Mol Cell Biol. 1992 Sep;12(9):4104–4111. doi: 10.1128/mcb.12.9.4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai P. K., Albers M. W., Chang H., Faber L. E., Schreiber S. L. Association of a 59-kilodalton immunophilin with the glucocorticoid receptor complex. Science. 1992 May 29;256(5061):1315–1318. doi: 10.1126/science.1376003. [DOI] [PubMed] [Google Scholar]
- Theodorakis N. G., Zand D. J., Kotzbauer P. T., Williams G. T., Morimoto R. I. Hemin-induced transcriptional activation of the HSP70 gene during erythroid maturation in K562 cells is due to a heat shock factor-mediated stress response. Mol Cell Biol. 1989 Aug;9(8):3166–3173. doi: 10.1128/mcb.9.8.3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanbuskirk A., Crump B. L., Margoliash E., Pierce S. K. A peptide binding protein having a role in antigen presentation is a member of the HSP70 heat shock family. J Exp Med. 1989 Dec 1;170(6):1799–1809. doi: 10.1084/jem.170.6.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voellmy R., Ahmed A., Schiller P., Bromley P., Rungger D. Isolation and functional analysis of a human 70,000-dalton heat shock protein gene segment. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4949–4953. doi: 10.1073/pnas.82.15.4949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelaw M. L., Hutchison K., Perdew G. H. A 50-kDa cytosolic protein complexed with the 90-kDa heat shock protein (hsp90) is the same protein complexed with pp60v-src hsp90 in cells transformed by the Rous sarcoma virus. J Biol Chem. 1991 Sep 5;266(25):16436–16440. [PubMed] [Google Scholar]
- Wiborg O., Pedersen M. S., Wind A., Berglund L. E., Marcker K. A., Vuust J. The human ubiquitin multigene family: some genes contain multiple directly repeated ubiquitin coding sequences. EMBO J. 1985 Mar;4(3):755–759. doi: 10.1002/j.1460-2075.1985.tb03693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B. J., Morimoto R. I. Transcription of the human hsp70 gene is induced by serum stimulation. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6070–6074. doi: 10.1073/pnas.82.18.6070. [DOI] [PMC free article] [PubMed] [Google Scholar]