Abstract
Rex, Christopher S., Julie C. Lauterborn, Ching-Yi Lin, Eniko A. Kramár, Gary A. Rogers, Christine M. Gall, and Gary Lynch. Restoration of long-term potentiation in middle-aged hippocampus after induction of brain-derived neurotrophic factor. J Neurophysiol 96: 677-685, 2006. First published May 17, 2006; doi:10.1152/jn.00336.2006. Restoration of neuronal viability and synaptic plasticity through increased trophic support is widely regarded as a potential therapy for the cognitive declines that characterize aging. Previous studies have shown that in the hippocampal CA1 basal dendritic field deficits in the stabilization of long-term potentiation (LTP) are evident by middle age. The present study tested whether increasing endogenous brain-derived neurotrophic factor (BDNF) could reverse this age-related change. We report here that in middle-aged (8- to 10-mo-old) rats, in vivo treatments with a positive AMPA-type glutamate receptor modulator both increase BDNF protein levels in the cortical telencephalon and restore stabilization of basal dendritic LTP as assessed in acute hippocampal slices 18 h after the last drug treatment. These effects were not attributed to enhanced synaptic transmission or to facilitation of burst responses used to induce LTP. Increasing extracellular levels of BDNF by exogenous application to slices of middle-aged rats was also sufficient to rescue the stabilization of basal dendritic LTP. Finally, otherwise stable LTP in ampakine-treated middle-aged rats can be eliminated by infusion of the extracellular BDNF scavenger TrkB-Fc. Together these results indicate that increases in endogenous BDNF signaling can offset deficits in the postinduction processes that stabilize LTP.
INTRODUCTION
Historically, age-related memory losses have been treated as sensitive indicators of late-in-life neuropathology, as is found in the early stages of Alzheimer’s disease or the precedent mild cognitive impairment. Recent work, however, confirms the everyday experience that deterioration of memory begins far in advance of old age. For example, an analysis of cohorts of human subjects from the third through the ninth decades led to the conclusion that “ . . . the magnitude of decline [of memory] is as great from 20 to 30 as it is from 70 to 80” (Park et al. 2002). Following on this, brain slice studies have shown that long-term potentiation (LTP), a presumed substrate of memory encoding, deteriorates, albeit only in particular groups of synapses, during the transition from young adulthood to early middle age in rat hippocampus (Rex et al. 2005).
To what degree can these regionally selective consequences of normal aging be corrected? One of the more widely embraced strategies for reversing the effects of brain aging involves increasing exposure to trophic factors (Mattson et al. 2004; Yuen and Mobley 1996), proteins with well-demonstrated capacities for sustaining and expanding neuronal connections (Conner et al. 2001; Mamounas et al. 1995), and promoting synaptic plasticity (for review see Bramham and Messaoudi 2005) in adult brain. Although most tests of this idea involve adding exogenous factors or new expression elements to the brain, pharmacological discoveries suggest means for increasing the production of endogenous growth factors in the absence of significant disturbances to behavior. It is well established that excitatory synaptic input regulates neuronal neurotrophin expression and that brain-derived neurotrophic factor (BDNF), in particular, is upregulated by even moderate increases in neuronal activity (Gall and Lauterborn 2000; Gall and Lynch 2005; Hall et al. 2000). Accordingly, the advent of drugs that positively modulate α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)-type glutamate receptors (“ampakines”), and thereby enhance excitatory transmission (Staubli et al. 1994a), provided a potential means for increasing neurotrophin signaling in adult brain. Experimental tests showed that ampakines do in fact significantly elevate BDNF expression in vitro and in freely moving animals (Lauterborn et al. 2000, 2003, Mackowiak et al. 2002). Other studies established that ampakines do not disrupt brain activity or behavior and can be administered for weeks without deleterious effect (Goff et al. 2001; Hampson et al. 1998). Thus there appear to be no outstanding concerns blocking the use of these drugs in a neurotrophin strategy for treating age-related brain disorders. The present study applied this approach to test 1) whether in vivo ampakine treatments can elevate BDNF protein levels in forebrain of middle-aged rats and 2) whether such increases in BDNF availability are accompanied by a reversal of regional, age-related deficits in the stabilization of LTP.
METHODS
Ampakine treatments and behavioral testing
All procedures were approved by the University of California Institutional Animal Care and Use Committee. The studies used middle-aged (8-10 mo; 450-600 g) adult male Sprague-Dawley rats (Charles Rivers Laboratories, Gilroy, CA) that matched the source and parameters used in our previous report of age effects on basal dendritic LTP (Rex et al. 2005). Rats were housed in pairs: one animal was randomly assigned to the ampakine-treatment regimen and the other was assigned to vehicle treatment. All animals received two intraperitoneal (ip) injections per day, at 8:00 to 9:00 am and 2:00 to 3:00 pm, for a total of 8 days (Fig. 1A). For the first 4 days, the rats received vehicle injections of normal saline plus 15-20% 2-hydroxypropyl-β-cyclodextrin (Sigma, St. Louis MO). After this period of acclimatization, rats assigned to drug treatment were injected for the next 4 days with the ampakine CX929 (5 mg/kg), whereas vehicle-assigned rats continued to receive injections of vehicle. Cohoused rat pairs were treated together: after each injection the pairs of rats were placed in an open field environment (consisting of a 110 × 55-cm opaque Plexiglas box containing compartments) for 20 min of exploration. Locomotor activity in the final cohort of rats was monitored and recorded on injection days 3 and 4 (before ampakine treatment) and days 5 and 6 (including the first 2 days of ampakine treatment) using an overhead-mounted video camera. Videos were converted to digital media and locomotor activity was traced by cursor and analyzed on a computer (in-house software, Python) for total distance traveled (in centimeters) and proportion of time active over the 20-min period. Data in graphs are represented as percentage activity during treatment sessions compared with pretreatment sessions (baseline) and significance was determined by two-tailed t-test. Data from days 4 (pretreatment) and 5 (treatment) were also binned in 5-min increments and analyzed to investigate motor activity throughout the 20-min observation period. Significance was assessed by two-way repeated-measures ANOVA for treatment effects across the 5-min bins (percentage of pretreatment baseline).
FIG. 1.
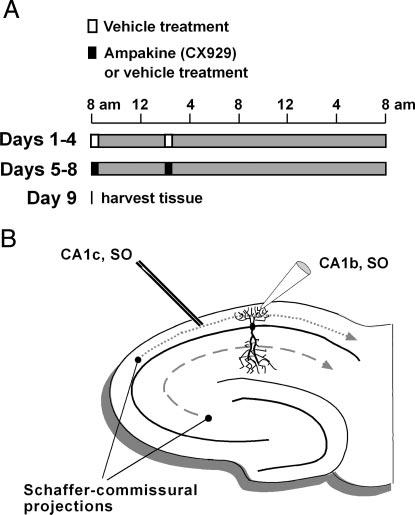
Schematics illustrating injection schedules and stratum (str.) oriens (SO) electrode placements used. A: time lines show injection schedules. For the first 4 days (days 1-4), all rats received vehicle injections (open boxes) twice daily at 8:00 am and 2:00 pm. During days 5-8, rats were injected by the same schedule with either the ampakine CX929 (5 mg/kg; closed boxes) for experimental rats or vehicle for control rats. Immediately after each injection, rat pairs were placed in an open-field environment for 20 min. Brain-derived neurotrophic factor (BDNF) protein and electrophysiological assays were carried out on day 9, 18 h after the last injection. B: schematic of a hippocampal slice showing the placement of stimulation (CA1c, SO) and recording (CA1b, SO) electrodes used to sample monosynaptic field excitatory postsynaptic potentials (fEPSPs) in SO of field CA1. Exclusive laminar arrangement of the Schaffer collateral/commissural projections to SO (short-dashed line) and str. radiatum (long-dashed line) allows for stimulation of the basal dendritic afferents without contributions from apical afferent fibers (and vice versa).
Hippocampal slice electrophysiology
At 18 h after the last ampakine injection, animals were killed and brains removed, and the two hemispheres were used for electrophysiology and protein measures, respectively. For electrophysiology, 350-μm-thick transverse slices through the midseptotemporal hippocampus were prepared on a vibrating tissue slicer (VT1000, Leica, Nusslock, Germany) and maintained at 30°C in an interface recording chamber as described (Rex et al. 2005) with continuous artificial cerebrospinal fluid (ACSF) perfusion at a rate of 60-70 ml/h. Slices equilibrated to the chamber for ≥1 h before recordings were initiated. Unless stated otherwise, a single glass electrode was placed within the most distal CA1b stratum (str.) oriens and was used to record field excitatory postsynaptic potentials (fEPSPs) from the basal dendrites of CA1 pyramidal cells (Fig. 1B). Responses were evoked by 0.03-Hz single-pulse stimulation of CA1c str. oriens using a twisted nichrome wire (65 μm) bipolar electrode (see Rex et al. 2005); with this stimulating electrode placement, fEPSPs would be expected to primarily reflect orthodromic stimulation of the highly topographic, basal dendritic Schaffer collateral/commissural afferents arising from pyramidal cells of field CA3a (Amaral and Witter 1985). Input-output curves and baseline measures were analyzed as described elsewhere (Rex et al. 2005). Synaptic potentiation was induced with theta-burst stimulation (TBS) (i.e., 10 bursts of 100-Hz stimulation with interburst intervals of 200 ms) (Kramar and Lynch 2003; Larson et al. 1986; Rex et al. 2005). Responses to individual theta bursts were analyzed to determine the burst area. To evaluate treatment effects on theta train facilitation, responses to each burst in the 10-burst theta train are presented as a percentage change from the area of the initial burst response (Kramar et al. 2004). Unless otherwise stated, group size values represent number of animals tested (values for each “n” within the group represent the mean of values from two to three slices from a given rat) and statistical significance was assessed using two-way repeated-measures ANOVA.
Drugs were administered to the bath by a second perfusion line connected to the main chamber input line after obtaining stable baseline fEPSPs for 10-20 min. BDNF (Chemicon, Temecula, CA) stock was prepared fresh immediately before slice preparation and diluted in ACSF before being added to the perfusion line. Stocks of recombinant TrkB-Fc and IgG-Fc chimeras (No., 688-TK and No., 110-HG; R&D Systems, Minneapolis, MN) (Cheng and Yeh 2005) were prepared in Tris-buffered saline containing 0.1% BSA and diluted in ACSF immediately before each experiment.
BDNF ELISA and Western blots
Hippocampi were dissected free and trimmed to include the dentate gyrus and hippocampus proper and homogenized in lysis buffer. Sample protein contents were measured (BioRad Protein Assay: BioRad Laboratories, Hercules, CA), volumes were adjusted to normalize μg/μl protein content, and then aliquots were processed for BDNF ELISA using the BDNF Emax Immunoassay System (Promega, Madison, WI) as described in detail elsewhere (Lauterborn et al. 2000). BDNF levels were determined relative to a standard curve constructed from measures of kit-supplied BDNF protein standards (0-500 pg BDNF protein) that were assayed simultaneously with experimental samples. Data are presented as means ± SE pg BDNF/100 μg of sample protein content.
To determine the effective TrkB-Fc dose for blocking BDNF signaling, Western blots assessed Trk phosphorylation in treated and control hippocampal slices (six slices/treatment condition) using a within-animal design. Tissues were homogenized in RIPA buffer, protein levels were measured using the BioRad Protein Assay (Bio-Rad Laboratories), and sample volumes were adjusted to normalize μg/μl protein content. Samples were then separated by 4-12% gradient PAGE and processed for Western blot analysis as described elsewhere (Lin et al. 2005) using the enhanced chemiluminescence ECL Plus Detection System (Amersham Biosciences, Buckinghamshire, UK) to visualize immunoreactive bands. Target proteins were probed using antibodies against total TrkB (anti-TrkB, #611641, BD Biosciences, San Jose, CA; diluted to 1:1,000) and phosphorylated Trk (1:1,000, anti-phospho TrkA tyr490, No., 9141; Cell Signaling Technology, Danvers, MA): the latter antiserum recognizes the conserved Trk phosphorylation/activation site within TrkB (He et al. 2002).
RESULTS
The first goal was to determine whether daily injections with the ampakine CX929 upregulate BDNF protein levels in middle-aged rat hippocampus. CX929 was used because it has a short half-life (Rogers, unpublished data) and previous experiments in cultured slices showed that relatively brief exposures to ampakines are sufficient to induce BDNF (Lauterborn et al. 2000, 2003). These slice studies also demonstrated that after a single exposure to ampakine BDNF protein levels remain elevated for 2-3 days before returning to normal, and that after intermittent ampakine treatment increases (measured 18-24 h after drug infusion) can be sustained for ≥5 days (Lauterborn et al. 2003; Lauterborn and Gall, unpublished observations). Based on these results, we chose to test for LTP 18 h after the last of four daily treatments with CX929 (Fig. 1A).
Pilot studies identified an appropriate dose range for CX929 effects on BDNF expression in vivo (i.e., it was determined that 1, 2.5, and 5 mg/kg increased BDNF proteins levels in young adult rats with the effects of 5 mg/kg being greatest). Middle-aged rats (8-10 mo old) received twice-daily injections of 5 mg/kg CX929 or vehicle for 4 days, after which their brains were collected for ELISA and electrophysiological measures (below). CX929 has a half-life in rats of about 15 min, so any measured changes in BDNF protein would represent effects that persisted long after the inducing condition was eliminated. Figure 2 summarizes BDNF protein levels in hippocampal tissue from ampakine- and vehicle-treated rats. As shown (Fig. 2A), BDNF levels in CX929-treated rats were significantly greater than those in vehicle-treated rats (4.08 ± 0.56 vs. 2.49 ± 0.16 pg/100 μg, respectively; P = 0.02). This accords with work showing that abbreviated ampakine treatments can increase hippocampal BDNF mRNA in aged rats in vivo (Lauterborn et al. 2000).
FIG. 2.

Ampakine treatment increases hippocampal BDNF protein levels without disturbing locomotor activity of middle-aged rats. Rats (8 to 10 mo old) were injected with 5 mg/kg CX929 or vehicle twice daily for 4 days and killed 18 h after the last injection. Hippocampi from left and right hemispheres were harvested for BDNF ELISA and electrophysiological assays, respectively. A: BDNF protein levels (plotted as pg BDNF/100 μg sample protein) were significantly greater in ampakine-vs. vehicle-treated rats (P = 0.02, 2-tailed t-test, n = 7/group; means ± SE). B: plots of group means (±SE) for measures of locomotor activity in vehicle-(open bars) and CX929-treated (closed bars) rats (n = 4/group). Values for distance traveled (left) and percent time moving (right) indicate no drug effect on locomotor activity (P>0.7, both measures). C: representative traces show movements of individual rats within the open field during the 20-min period after vehicle or CX929 injection. Straight lines within the field show the position of a raised platform (left side) and 2 triangular partitions (right side).
In the present experiments, drugs were administered to well-handled rats engaged in a 20-min period of exploration and social interaction in a large open field after each injection. In addition to testing for neurotrophin increases in naturalistic circumstances, this procedure allowed for tests of whether the ampakine disturbed complex behavior during the brief period in which it was present at the highest levels. It should be noted that there is an extensive literature showing that hippocampal lesions have pronounced effects on exploratory activity (Campbell et al. 1971; Kimble 1963) and social behavior (Bannerman et al. 2001). As shown in Fig. 2, B and C, there were no differences between vehicle- and CX929-treated animals in measures of distance traveled (88.7 ± 14.1 vs. 93.1 ± 18.5% of baseline for vehiclevs. ampakine-treated; P>0.7, two-tailed t-test) and time spent traveling (94.1 ± 7.2 vs. 94.5 ± 14.2%, P>0.8) during the period of social exploration. Further analysis of these behaviors during shorter time intervals (i.e., for each 5-min increment during the 20-min observation period) failed to detect transient alterations in locomotor behavior as a result of ampakine injection (P>0.5 for distance traveled and percentage time moving, two-way repeated-measures ANOVA). Results presented in Fig. 2 are from rats allowed to explore in pairs including one ampakine- and one vehicle-treated rat. Similarly, behavioral testing of pairs of like-treated rats (i.e., two vehicle-treated or two ampakine-treated rats tested together) revealed no drug effect on distance traveled (92.2 ± 12.7 vs. 98.3 ± 13.1% for vehicle- and ampakine-treated rats, respectively; P>0.6, two-tailed t-test) or time spent traveling (89.3 ± 9.3 vs. 95.9 ± 13.8%; P>0.7).
Figure 3 describes the percentage LTP obtained in str. oriens of slices prepared from vehicle-versus ampakine-treated middle-aged rats. In agreement with an earlier report (Rex et al. 2005), TBS-induced potentiation of Schaffer collateral synapses in the CA1 basal dendritic field did not stabilize in slices from vehicle-treated middle-aged rats, as it does in slices from young adult rats, but instead decayed nearly to baseline within 60 min (117 ± 5% of baseline; mean ± SE). As in our prior study, and as indicated in Fig. 3B, there was little variability in response properties in slices from vehicle-treated middle-aged rats; i.e., slices from all rats tested showed impaired LTP stabilization. Very different results were obtained in slices from ampakine-treated rats with elevated BDNF levels: in these cases, potentiation was persistent, with fEPSP measures being 149 ± 7% of baseline at 60 min after TBS. The difference in percentage LTP between slices from vehicle- and ampakine-treated rats was significant (P < 0.001 for minutes 50-60, n = 12/group).
FIG. 3.
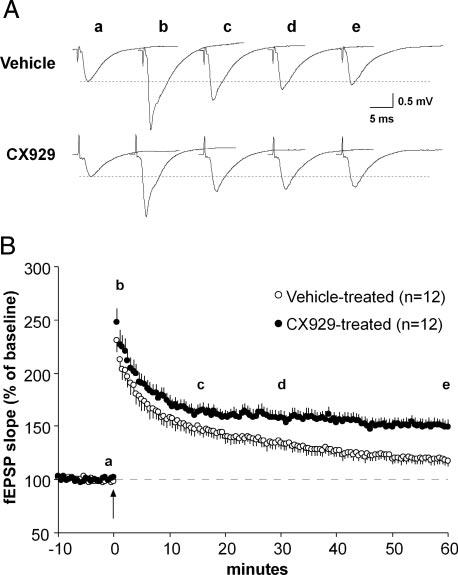
Ampakine pretreatment rescues long-term potentiation (LTP) stabilization in middle-aged rats. Basal dendritic fEPSPs (elicited by stimulation of the Schaffer collateral/commissural afferents to CA1b SO as illustrated in Fig. 1B) were compared for slices from middle-aged rats that received CX929 or vehicle injections for 4 days. Slices were prepared 18 h after the last injections. A: representative fEPSP traces (a-e) collected during the baseline period and 1, 15, 30, and 60 min (from left to right) after theta-burst stimulation from vehicle- and CX929-treated rats. Figures are averages of 3 consecutive traces. B: plot of group mean (±SE) fEPSP slopes recorded in slices from ampakine-treated (filled circle) and vehicle-treated (open circle) rats. Theta bursts were delivered (time 0, arrow) after establishing that baseline responses (3/min) were constant over 10-20 min. Letters a-e indicate the time points for traces shown in A. As shown, slices from ampakine- and vehicle-treated rats had approximately equal degrees of potentiation immediately after TBS. Responses in slices from CX929-treated rats settled to a stable level by 20 min post-TBS and persisted there through 60 min of testing. In contrast, responses in slices from vehicle-treated rats steadily declined toward baseline values from the initial peak: percentage potentiation at 60 min post-TBS was significantly greater in the ampakine-treatment group than in controls (P < 0.001 for minutes 50-60 post-TBS).
In a previous study (Rex et al. 2005) we found that LTP in the apical dendrites (str. radiatum) of control middle-aged rats was not detectably different from that described for young adult animals and thus does not exhibit the stabilization deficit associated with the basal dendrites. For Schaffer collateral innervation of the apical field, daily ampakine treatment had no significant effect on the magnitude or stability of potentiation (140 ± 11% for control and 146 ± 12% for experimental rats at 60 min after stimulation; P>0.3 for minutes 50-60; Fig. 4A). Moreover, there were no differences between slices from vehicle- and ampakine-treated animals for str. radiatum fEPSP input/output curves (Fig. 4, B and C) or measures of burst responses (P>0.4 and P>0.5, respectively; two-way repeated-measures ANOVA). It should be noted that the presence of seemingly normal LTP in the apical dendrites argues against the possibility that the deficits in LTP stabilization within the basal dendritic field reflect a general problem with middle-aged hippocampus or slices prepared from it.
FIG. 4.

Comparison of fEPSPs and theta-burst responses in slices prepared from middle-aged rats that had received vehicle or ampakine injections. Plots A-C show results from recordings within str. radiatum and plots D-G show results from recordings within str. oriens. A: theta bursts were delivered to the Schaffer-commissural projections to the apical dendrites (str. radiatum) of field CA1. Graph summarizes the mean sizes (±SEs) of fEPSPs collected from hippocampal slices prepared from CX929-treated (closed circles) or vehicle-treated (open circles) middle-aged rats. There were no reliable differences in percentage potentiation between the two groups (P>0.3 for minutes 50-60). B and C: input/output (I/O) curves were generated for slices represented in A by stimulating the Schaffer-commissural afferents to CA1 apical dendrites at a constant current (30 μA) with varying pulse durations. Representative traces (B) from slices of vehicle- vs. CX929-treatment groups were not detectably different. Group data (C) showed no effect of CX929 on fEPSP amplitudes (P>0.4). D and E: I/O curves were generated for basal dendritic, Schaffer-commissural fEPSP responses of slices represented in Fig. 3. D: representative traces generated from increasing stimulus duration steps were not detectably different for slices from vehicle- and CX929-treated animals. E: group I/O data from str. oriens showed no effect of CX929 on fEPSP amplitude (P>0.5, n = 12/group). F and G: size and waveforms of theta-burst responses within the CA1 basal dendrites (str. oriens) were compared in vehicle- and ampakine-treated middle-aged rats (same as represented in D and E). F: records from 5 slices were averaged to produce representative responses to the first and fourth theta bursts. G: graph showing facilitation of str. oriens theta-burst responses during a 10-burst train for vehicle-treated (open bars) and CX929-treated (closed bars) rats. Response size was calculated as the percentage increase of the area of measured burst response over the area of the first burst response. Plot shows group means (±SE) for bursts 2-10. Within-train burst-response profile was not statistically different for slices from ampakine- and vehicle-treated rats (n = 12/group).
Increasing the size of theta-burst responses, either by enhancing synaptic currents or reducing factors that depress the responses during a train, can dramatically facilitate the induction of LTP (Arai and Lynch 1992). However, such effects do not appear to be responsible for the restoration of basal dendritic potentiation described above. Input/output curves for str. oriens fEPSPs (Fig. 4, D and E) were virtually identical for slices from ampakine- and vehicle-treated middle-aged rats (P>0.5; two-way repeated-measures ANOVA), indicating that a given-sized stimulation pulse elicited about the samesized response in the two groups of slices. Careful examination of the area of theta-burst responses also failed to identify between-group differences (Fig. 4, F and G). The absolute size of the initial burst response was not different in slices prepared from vehicle-versus ampakine-treated rats (P>0.8, two-tailed t-test) nor was the degree to which the burst responses facilitated within a train (60 ± 7%; mean burst facilitation for bursts 2-10 for controls vs. 65 ± 7% for experimental slices; P>0.5, two-way repeated-measures ANOVA) (Fig. 4G). Finally, the initial degree of potentiation recorded in the first minute after the theta-burst train was comparable for the two groups of slices; the mean for minute 1 was 222 ± 11% of baseline for controls and 237 ± 13% for slices from ampakine-treated rats. This observation reinforces the conclusion from the analysis of theta burst that the inducing conditions for LTP were comparable in the two groups of slices.
It should be noted that the shape of the composite response to a theta burst depends on frequency facilitation across four fEPSPs, whereas the size of the responses is influenced by inhibitory postsynaptic potentials (IPSPs) (Larson and Lynch 1986) and N-methyl-d-aspartate (NMDA)-receptor-mediated currents (Larson and Lynch 1988). The within-train facilitation effect illustrated in Fig. 4, F and G is in part governed by afterhyperpolarizing potentials (Arai and Lynch 1992; Kramár et al. 2004). Therefore the absence of differences in burst responses or theta-train facilitation between ampakine-treated and control groups indicates that the restoration of LTP in the former slices cannot be attributed to changes in these primary physiological variables.
Results from multiple studies using BDNF or its antagonists provide strong evidence that the neurotrophin promotes the formation of LTP and does so through the TrkB receptor (Chen et al. 1999; Figurov et al. 1996; Kang et al. 1997; Lu et al. 2005; Minichiello et al. 2002). Thus rather than acting directly on the mechanisms that consolidate LTP, ampakines could offset age-related losses in potentiation by the elevated BDNF levels described here. If so, then infusion of exogenous BDNF should restore stable potentiation to the basal dendrites of middle-aged slices, whereas antagonists of endogenous BDNF signaling should eliminate the rescued LTP in slices from ampakine-treated rats. Figure 5 summarizes a test of the first of these predictions. In accord with earlier reports (Kramar et al. 2004), bath application of 2 nM BDNF did not alter the slope of Schaffer collateral fEPSPs in response to single-pulse stimulation (Fig. 5, A and B). However, theta bursts delivered 50 min after the onset of BDNF infusion induced robust and persistent LTP that was significantly greater (P < 0.05 for minutes 50-60) than potentiation in untreated slices from the same rats.
FIG. 5.
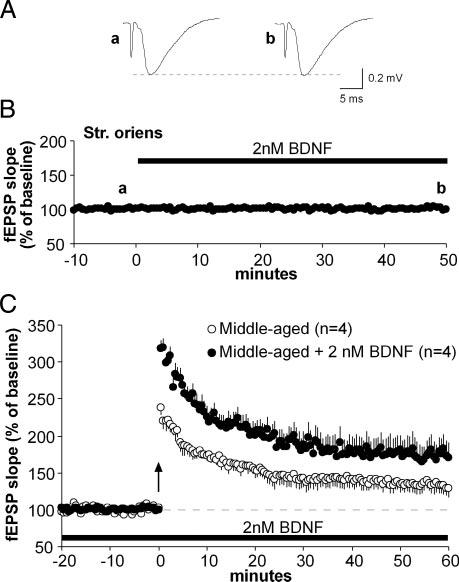
Exogenous BDNF promotes the formation of basal dendritic LTP in middle-aged rats. Schaffer-commissural responses were recorded from CA1b str. oriens in hippocampal slices of untreated middle-aged (8-10 mo) rats. A: representative traces of responses to single-pulse stimulation before (a) and 50 min after (b) the onset of bath application of 2 nM BDNF. Each trace is the average of 3 responses at the indicated time points (below). B: plot shows group means (±SE) of fEPSP slopes. BDNF was infused (black bar) for 50 min after a 10- to 20-min period of stable baseline recording. Time points of representative traces in A are indicated above trace (a, b). C: LTP was induced using theta-burst stimulation (time 0, arrow) in the same slices represented above (closed circles). Infusion of 2 nM BDNF continued through 60 min after stimulation. Compared with control slices (open circles) from middle-aged animals, BDNF-treated slices showed significantly greater potentiation (P < 0.05; n = number slices tested) that appeared stable at a level comparable to that found in slices from ampakine-treated rats (see Fig. 3).
The BDNF scavenger TrkB-Fc, a fusion construct that sequesters extracellular BDNF (Davis et al. 1994), was used to test whether—as predicted— competitively blocking the actions of released, endogenous BDNF eliminates the positive effects of daily ampakine treatment on basal dendritic LTP. Effective concentrations of TrkB-Fc were established using BDNF-induced Trk phosphorylation as a marker. Treatment of hippocampal slices with 60 ng/ml BDNF significantly increased Trk phosphorylation (3,888 ± 233 vs. 2,725 ± 35 optical density units for BDNF-treated vs. untreated slices, means ± SE; P < 0.05, paired t-test). TrkB-Fc inhibition of this effect was dose dependent (P < 0.001; one-way ANOVA; n = 10) with robust suppression at 1.0 μg/ml (Fig. 6, A and B), whereas blots that were stripped and reprobed with anti-TrkB showed no treatment effects on total TrkB protein levels (P>0.9, one-way ANOVA). Treatment of hippocampal slices with 1.0 μg/ml TrkB-Fc alone did not influence basal phospho-Trk levels (data not shown) and, when applied to slices from ampakine-treated middle-aged rats, did not influence baseline fEPSPs through 30 min (Fig. 6C). However, as shown in Fig. 6D, 1.0 μg/ml TrkB-Fc blocked the restoration of LTP stabilization in ampakine-treated middle-aged rats. In these cases, TBS induced an initial potentiation that declined to near baseline levels over 60 min. In contrast, potentiation in slices from ampakine-treated middle-aged rats treated with the control chimeric immunoglobulin IgG-Fc (1.0 μg/ml) was stable through 1 h and was significantly greater than that in 1.0 μg/ml TrkB-Fc-treated slices (P < 0.05 for comparison of fEPSP slopes for 50-60 min post-TBS). TrkB-Fc at a lower concentration (0.2 μg/ml) caused a modest degradation of the potentiation as assessed at 50-60 min (Fig. 6D) but differences between this group and the IgG-Fc-treated slices were not statistically significant. Finally, TrkB-Fc (1.0 μg/ml) failed to significantly attenuate the reduced LTP found in slices from control (vehicle-treated) middle-aged animals: Percentage potentiation (group fEPSP slope means ± SE; % of baseline) at 60 min was 117 ± 8% (n = 3 slices) for control middle-aged slices and 117 ± 10% (n = 4) for comparable slices pretreated (as described above) with the scavenger (P>0.6, two-way repeated-measures ANOVA for minutes 50-60). The initial level of potentiation was also equivalent in the two groups. The absence of a TrkB-Fc effect on LTP in the middle-aged slice indicates that the age-related deficit is specific for the neurotrophin-dependent component of the potentiation.
FIG. 6.
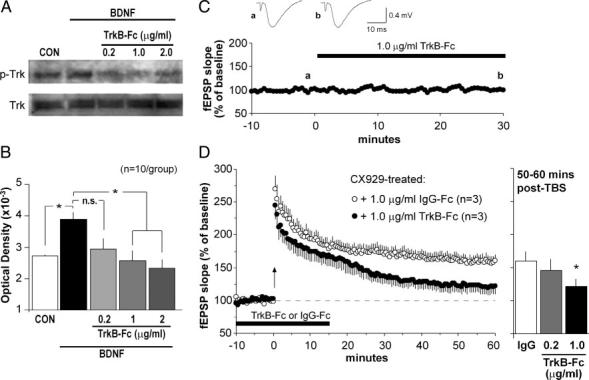
Rescue of LTP by ampakine pretreatment is reversed by the BDNF scavenger TrkB-Fc. A and B: quantitative analysis of phosphorylated Trk protein (pTrk) levels in adult hippocampal slices was used to assess an effective dose range for TrkB-Fc, a scavenger of extracellular BDNF. A: Western blots showing (top blot) that slices receiving 30-min infusions of 60 ng/ml BDNF had greater pTrk levels than did untreated control (CON) slices, and that pTrk levels were reduced by TrkB-Fc in a dose-dependent fashion (right 3 lanes show samples from slices treated with 60 ng/ml BDNF plus the TrkB-Fc dose indicated in μg/ml). Treatments had no effect on total Trk protein levels (bottom blot). B: plot shows mean (±SE) pTrk band densities for 10 Western blot experiments of the type described in A (*P < 0.05; n.s., not significant). C: effects of TrkB-Fc (1.0 μg/ml; black bar) on baseline synaptic responses were assessed using Schaffer-commissural evoked potentials recorded in CA1 str. oriens. This plot, and representative fEPSP traces (inset), indicate that TrkB-Fc infusion did not alter responses to single-pulse stimulation. D: TBS was delivered (time 0, arrow) to slices from CX929-treated rats that had been infused with either 1.0 μg/ml TrkB-Fc (closed circles) or 1 μg/ml IgG-Fc (open circles) for 30 min (black bar); the plot summarizes mean (± SE) values for fEPSP slopes. As shown, TrkB-Fc blocked the stabilization of LTP leading to a slow decay in potentiation, whereas infusion of the control IgG-Fc had no effect. Bar graph to the right describes the percentage potentiation averaged for minutes 50-60 post-TBS for slices from ampakine-treated rats infused with 1 μg/ml IgG-Fc, 0.2 μg/ml TrkB-Fc, or 1 μg/ml TrkB-Fc (n = 3/group for all plots; *P < 0.05 for comparison to IgG group).
DISCUSSION
The above results constitute the first evidence that ampakines can be used to elevate endogenous BDNF protein levels in older rats, show that this effect occurs without evident disturbances to ongoing behavior, and establish that it can be achieved with surprisingly brief exposures to the drugs. It thus appears that a several minutes-long period, during which postsynaptic responses generated by behavior are enhanced, is adequate to produce substantial, long-lasting increases in BDNF protein. This accords with evidence that short bursts of excitatory afferent drive can significantly increase BDNF gene expression (Castrén et al. 1993) and that induced increases in BDNF protein persist for days after transient increases in mRNA (Gall and Lauterborn 2000; Nawa et al. 1995).
Taken together, these findings suggest a strategy for regulating neurotrophin levels in adult brain that is minimally disruptive with regard to physiology and behavior. There are no evident barriers to chronic ampakine treatment because the drugs, albeit in versions less potent than the one used here, have been administered for weeks to animals (Hampson et al. 1998) and humans (Goff et al. 2001) without notable side effects. Ampakines could thus be used to offset the declines in BDNF described for middle-aged rodents (Gooney et al. 2004; Hattiangady et al. 2005) and primates (Collier et al. 2005; Lommatzsch et al. 2005). Beyond this, elevating endogenous BDNF levels might reproduce the beneficial effects obtained with infusion of the exogenous neurotrophin (Conner et al. 2001; Patterson et al. 1996; see also O’Neill et al. 2004). A first step in testing these ideas will be to determine how large and reliable an increase can be achieved with daily, systemic ampakine injections and whether the effect can be maintained for longer periods than those tested in the present experiments. Possibly related to the effects on BDNF, ampakine treatment produced a dramatic improvement in the stability of LTP in middle-aged slices at the one site (basal dendrites of field CA1) at which deficits have so far been identified (Rex et al. 2005). It seems likely that future studies will identify other regions with impairments in synaptic plasticity by early middle age and thereby provide targets for tests of the generality of results described here. Given that physiological testing in the present studies was carried out well after the last ampakine treatment, it is likely that the observed restoration of enduring LTP was attributable to the still-present elevations in the neurotrophin. BDNF promotes LTP in multiple ways (Barco et al. 2005; Kramar et al. 2004). It causes a substantial increase in thetaburst responses, in part at least by blocking potassium channels that normally reduce within-train response facilitation (Kramar et al. 2004). However, there was no evidence for such an effect in the present studies; responses to theta-stimulation trains were comparable in control and ampakine-treated cases. This suggests that the ampakine-induced increase in BDNF does not result in tonic stimulation of TrkB receptors or the downstream kinases responsible for governing postsynaptic responses to theta bursts (for discussion see Kramar et al. 2004).
Alternatively, increasing BDNF levels and signaling may have specifically augmented postinduction events that stabilize LTP. Recent studies showed that theta-burst stimulation causes BDNF release (Balkowiec and Katz 2002), with elevated levels persisting ≤12 min after the theta train (Aicardi et al. 2004). This observation raises the possibility that BDNF released during theta stimulation promotes cellular processes that stabilize LTP during the minutes after afferent stimulation. This interpretation is supported by present results showing that TrkB-Fc potently blocked LTP stabilization in slices from ampakine-treated animals, without significantly affecting induction and initial expression. It is also consistent with the somewhat different effects of exogenous BDNF, on one hand, and ampakine-induced increases in endogenous BDNF on the other, on middle-aged, basal dendritic LTP. Both treatments restored LTP stabilization, but exogenous BDNF also enhanced the immediate, posttetanic potentiation, whereas CX929 pretreatment did not. This may reflect the fact that exogenous BDNF was applied 50 min before stimulation and was therefore available to influence processes (e.g., NMDA receptor and potassium channel phosphorylation states, postsynaptic scaffold associations) (Iki et al. 2005; Kramar et al. 2004; Lin et al. 1998) before and during TBS, whereas the endogenous neurotrophin, released by TBS, would be available to influence processes only after the initial induction events. These results support the view that release of endogenous BDNF is necessary for the expression of restored LTP in ampakine-treated rats but do not establish that greater release of the neurotrophin is directly responsible for that restoration. However, multiple, independent lines of evidence support the hypothesis and it is certainly the most parsimonious explanation for the observed effect of the ampakine. Additional experiments will test the effects of blocking the increase in BDNF expression with ampakine treatments in vivo, although such studies will require controls for the baseline effects of reducing neurotrophin levels.
Independent of mechanism, the present results show for the first time that daily ampakine treatments can have enduring effects that reduce a deleterious effect of early aging (i.e., the lost capacity for stable basal dendritic LTP) on hippocampal physiology. Previous studies showed that the acute actions of these drugs can also restore potentiation to the basal dendrites (Rex et al. 2005) and offset age-related deterioration of spatial learning (Granger et al. 1996) in rats, and substantially improve memory retention scores in aged humans (Lynch et al. 1997). However, these earlier experiments assessed LTP or memory shortly after ampakine administration and the improvements were interpreted as being reflections of the acute effects of the drugs (Arai et al. 2004; Staubli et al. 1994b). A therapeutic strategy based on these earlier findings would thus require maintenance of effective drug levels throughout most of the subject’s waking hours. Our current findings point to the unexpected possibility that intermittent, minutes-long exposures to appropriate ampakines can restore synaptic plasticity on a continuous basis. There are at present no experimental data addressing the prediction that daily treatments with short-half-life ampakines, and the restoration of LTP as reported here, will ameliorate age-related memory losses in tests carried out in the absence of the drugs. Retention deficits that emerge during middle age in rats (Erickson and Barnes 2003; Foster 1999) provide reasonable targets for a first test of this idea.
ACKNOWLEDGMENTS
The authors thank S. T. Cheng for assistance with ELISA assays and Cortex Pharmaceuticals (Irvine, CA) for generously providing the CX929.
Footnotes
This research was supported by National Institutes of Health Grants NS-45260, AG-00358, and NS-051823. C. S. Rex was supported, in part, by National Institute of Mental Health Training Grant 5-T32-MH-14599-27.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Aicardi G, Argilli E, Cappello S, Santi S, Riccio M, Thoenen H, Canossa M. Induction of long-term potentiation and depression is reflected by corresponding changes in secretion of endogenous brain-derived neurotrophic factor. 2004;101:15788–15792. doi: 10.1073/pnas.0406960101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral DG, Witter MP. Hippocampal formation. In: Paxinos G, editor. The Rat Nervous System. Academic Press; Orlando, FL: 1985. [Google Scholar]
- Arai A, Lynch G. Factors regulating the magnitude of long-term potentiation induced by theta pattern stimulation. Brain Res. 1992;598:173–184. doi: 10.1016/0006-8993(92)90181-8. [DOI] [PubMed] [Google Scholar]
- Arai AC, Xia YF, Suzuki E. Modulation of AMPA receptor kinetics differentially influences synaptic plasticity in the hippocampus. Neuroscience. 2004;123:1011–1024. doi: 10.1016/j.neuroscience.2003.10.033. [DOI] [PubMed] [Google Scholar]
- Balkowiec A, Katz DM. Cellular mechanisms regulating activity-dependent release of native brain-derived neurotrophic factor from hippocampal neurons. J Neurosci. 2002;22:10399–10407. doi: 10.1523/JNEUROSCI.22-23-10399.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannerman DM, Lemaire M, Beggs S, Rawlins JN, Iversen SD. Cytotoxic lesions of the hippocampus increase social investigation but do not impair social-recognition memory. Exp Brain Res. 2001;138:100–109. doi: 10.1007/s002210100687. [DOI] [PubMed] [Google Scholar]
- Barco A, Patterson S, Alarcon JM, Gromova P, Mata-Roig M, Morozov A, Kandel ER. Gene expression profiling of facilitated L-LTP in VP16-CREB mice reveals that BDNF is critical for the maintenance of LTP and its synaptic capture. Neuron. 2005;48:123–137. doi: 10.1016/j.neuron.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Bramham CR, Messaoudi E. BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Prog Neurobiol. 2005;76:99–125. doi: 10.1016/j.pneurobio.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Campbell BA, Ballantine P, Lynch G. Hippocampal control of behavioral arousal: duration of lesion effects and possible interactions with recovery after frontal cortical damage. Exp Neurol. 1971;33:159–170. doi: 10.1016/0014-4886(71)90110-5. [DOI] [PubMed] [Google Scholar]
- Castrén E, Pitkänen M, Sirviö J, Parsadanian A, Lindholm D, Thoenen H, Riekkinen PJ. The induction of LTP increases BDNF and NGF mRNA but decreases NT-3 mRNA in the dentate gyrus. Neuroreport. 1993;4:895–898. doi: 10.1097/00001756-199307000-00014. [DOI] [PubMed] [Google Scholar]
- Chen G, Kolbeck R, Barde YA, Bonhoeffer T, Kossel A. Relative contribution of endogenous neurotrophins in hippocampal long-term potentiation. J Neurosci. 1999;19:7983–7990. doi: 10.1523/JNEUROSCI.19-18-07983.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Q, Yeh HH. PLCgamma signaling underlies BDNF potentiation of Purkinje cell responses to GABA. J Neurosci Res. 2005;79:616–627. doi: 10.1002/jnr.20397. [DOI] [PubMed] [Google Scholar]
- Collier TJ, Dung Ling Z, Carvey PM, Fletcher-Turner A, Yurek DM, Sladek JR, Jr, Kordower JH. Striatal trophic factor activity in aging monkeys with unilateral MPTP-induced parkinsonism. Exp Neurol. 2005;191(Suppl 1):S60–S67. doi: 10.1016/j.expneurol.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Conner JM, Darracq MA, Roberts JD, Tuszynski MH. Nontropic actions of neurotrophins: subcortical nerve growth factor gene delivery reverses age-related degeneration of primate cortical cholinergic innervation. Proc Natl Acad Sci USA. 2001;98:1941–1946. doi: 10.1073/pnas.98.4.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis S, Gale NW, Aldrich TH, Maisonpierre PC, Lhotak V, Pawson T, Goldfarb M, Yancopoulos GD. Ligands for EPH-related receptor tyrosine kinases that require membrane attachment or clustering for activity. Science. 1994;266:816–819. doi: 10.1126/science.7973638. [DOI] [PubMed] [Google Scholar]
- Erickson CA, Barnes CA. The neurobiology of memory changes in normal aging. Exp Gerontol. 2003;38:61–69. doi: 10.1016/s0531-5565(02)00160-2. [DOI] [PubMed] [Google Scholar]
- Figurov A, Pozzo-Miller LD, Olafsson P, Wang T, Lu B. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature. 1996;381:706–709. doi: 10.1038/381706a0. [DOI] [PubMed] [Google Scholar]
- Foster TC. Involvement of hippocampal synaptic plasticity in age-related memory decline. Brain Res Rev. 1999;30:236–249. doi: 10.1016/s0165-0173(99)00017-x. [DOI] [PubMed] [Google Scholar]
- Gall CM, Lauterborn JC. Regulation of BDNF expression: multifaceted, region-specific control of a neuronal survival factor in the adult CNS. In: Mocchetti I, editor. Neurobiology of the Neurotrophins. FP Graham; Johnson City, TN: 2000. [Google Scholar]
- Gall CM, Lynch G. Consolidation: a view from the synapse. In: Stanton PK, Bramham C, Scharfman HE, editors. Synaptic Plasticity and Transynaptic Signaling. Springer Science; New York: 2005. [Google Scholar]
- Goff DC, Leahy L, Berman I, Posever T, Herz L, Leon AC, Johnson SA, Lynch G. A placebo-controlled pilot study of the ampakine CX516 added to clozapine in schizophrenia. J Clin Psychopharmacol. 2001;21:484–487. doi: 10.1097/00004714-200110000-00005. [DOI] [PubMed] [Google Scholar]
- Gooney M, Messaoudi E, Maher FO, Bramham CR, Lynch MA. BDNF-induced LTP in dentate gyrus is impaired with age: analysis of changes in cell signaling events. Neurobiol Aging. 2004;25:1323–1331. doi: 10.1016/j.neurobiolaging.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Granger R, Deadwyler S, Davis M, Moskovitz B, Kessler M, Rogers G, Lynch G. Facilitation of glutamate receptors reverses an age-associated memory impairment in rats. Synapse. 1996;22:332–337. doi: 10.1002/(SICI)1098-2396(199604)22:4<332::AID-SYN4>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Hall J, Thomas KL, Everitt BJ. Rapid and selective induction of BDNF expression in the hippocampus during contextual learning. Nat Neurosci. 2000;3:533–535. doi: 10.1038/75698. [DOI] [PubMed] [Google Scholar]
- Hampson RE, Rogers G, Lynch G, Deadwyler SA. Facilitative effects of the ampakine CX516 on short-term memory in rats: enhancement of delayed-nonmatch-to-sample performance. J Neurosci. 1998;18:2740–2747. doi: 10.1523/JNEUROSCI.18-07-02740.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattiangady B, Rao MS, Shetty GA, Shetty AK. Brain-derived neurotrophic factor, phosphorylated cyclic AMP response element binding protein and neuropeptide Y decline as early as middle age in the dentate gyrus and CA1 and CA3 subfields of the hippocampus. Exp Neurol. 2005;195:353–371. doi: 10.1016/j.expneurol.2005.05.014. [DOI] [PubMed] [Google Scholar]
- He XP, Minichiello L, Klein R, McNamara JO. Immunohistochemical evidence of seizure-induced activation of trkB receptors in the mossy fiber pathway of adult mouse hippocampus. J Neurosci. 2002;22:7502–7508. doi: 10.1523/JNEUROSCI.22-17-07502.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iki J, Inoue A, Bito H, Okabe S. Bi-directional regulation of postsynaptic cortactin distribution by BDNF and NMDA receptor activity. Eur J Neurosci. 2005;22:2985–2994. doi: 10.1111/j.1460-9568.2005.04510.x. [DOI] [PubMed] [Google Scholar]
- Kang H, Welcher AA, Shelton D, Schuman EM. Neurotrophins and time: different roles for TrkB signaling in hippocampal long-term potentiation. Neuron. 1997;19:653–664. doi: 10.1016/s0896-6273(00)80378-5. [DOI] [PubMed] [Google Scholar]
- Kimble DP. The effects of bilateral hippocampal lesions in rats. J Comp Physiol Psychol. 1963;56:273–283. doi: 10.1037/h0048903. [DOI] [PubMed] [Google Scholar]
- Kramar EA, Lin B, Lin CY, Arai AC, Gall CM, Lynch G. A novel mechanism for the facilitation of theta-induced long-term potentiation by brain-derived neurotrophic factor. J Neurosci. 2004;24:5151–5161. doi: 10.1523/JNEUROSCI.0800-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramar EA, Lynch G. Developmental and regional differences in the consolidation of long-term potentiation. Neuroscience. 2003;118:387–398. doi: 10.1016/s0306-4522(02)00916-8. [DOI] [PubMed] [Google Scholar]
- Larson J, Lynch G. Induction of synaptic potentiation in hippocampus by patterned stimulation involves two events. Science. 1986;232:985–988. doi: 10.1126/science.3704635. [DOI] [PubMed] [Google Scholar]
- Larson J, Lynch G. Role of N-methyl-d-aspartate receptors in the induction of synaptic potentiation by burst stimulation patterned after the hippocampal theta-rhythm. Brain Res. 1988;441:111–118. doi: 10.1016/0006-8993(88)91388-1. [DOI] [PubMed] [Google Scholar]
- Larson J, Wong D, Lynch G. Patterned stimulation at the theta frequency is optimal for the induction of hippocampal long-term potentiation. Brain Res. 1986;368:347–350. doi: 10.1016/0006-8993(86)90579-2. [DOI] [PubMed] [Google Scholar]
- Lauterborn JC, Lynch G, Vanderklish P, Arai A, Gall CM. Positive modulation of AMPA receptors increases neurotrophin expression by hippocampal and cortical neurons. J Neurosci. 2000;20:8–21. doi: 10.1523/JNEUROSCI.20-01-00008.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauterborn JC, Truong GS, Baudry M, Bi X, Lynch G, Gall CM. Chronic elevation of brain-derived neurotrophic factor by ampakines. J Pharmacol Exp Ther. 2003;307:297–305. doi: 10.1124/jpet.103.053694. [DOI] [PubMed] [Google Scholar]
- Lin CY, Lynch G, Gall CM. AMPA receptor stimulation increases alpha5beta1 integrin surface expression, adhesive function and signaling. J Neurochem. 2005;94:531–546. doi: 10.1111/j.l471-4159.2005.03203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Wu K, Levine ES, Mount HT, Suen PC, Black IB. BDNF acutely increases tyrosine phosphorylation of the NMDA receptor subunit 2B in cortical and hippocampal postsynaptic densities. Mol Brain Res. 1998;55:20–27. doi: 10.1016/s0169-328x(97)00349-5. [DOI] [PubMed] [Google Scholar]
- Lommatzsch M, Zingler D, Schuhbaeck K, Schloetcke K, Zingler C, Schuff-Werner P, Virchow JC. The impact of age, weight and gender on BDNF levels in human platelets and plasma. Neurobiol Aging. 2005;26:115–123. doi: 10.1016/j.neurobiolaging.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Lu B, Pang PT, Woo NH. The yin and yang of neurotrophin action. Nat Rev Neurosci. 2005;6:203–214. doi: 10.1038/nrn1726. [DOI] [PubMed] [Google Scholar]
- Lynch G, Granger R, Ambros-Ingerson J, Davis CM, Schehr R, Kessler M. Evidence that a positive modulator of AMPA-type glutamate receptors improves delayed recall in aged humans. Exp Neurol. 1997;145:89–92. doi: 10.1006/exnr.1997.6447. [DOI] [PubMed] [Google Scholar]
- Mackowiak M, O'Neill MJ, Hicks CA, Bleakman D, Skolnick P. An AMPA receptor potentiator modulates hippocampal expression of BDNF: an in vivo study. Neuropharmacology. 2002;43:1–10. doi: 10.1016/s0028-3908(02)00066-7. [DOI] [PubMed] [Google Scholar]
- Mamounas LA, Blue ME, Siuciak JA, Altar CA. Brain-derived neurotrophic factor promotes the survival and sprouting of serotonergic axons in rat brain. J Neurosci. 1995;15:7929–7939. doi: 10.1523/JNEUROSCI.15-12-07929.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson M, Maudsley S, Martin B. A neural signaling triumvirate that influences ageing and age-related disease: insulin/IGF-1, BDNF and serotonin. Ageing Res Rev. 2004;3:445–464. doi: 10.1016/j.arr.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Minichiello L, Calella A, Medina D, Bonhoeffer T, Klein R, Korte M. Mechanism of TrkB-mediated hippocampal long-term potentiation. Neuron. 2002;36:121–137. doi: 10.1016/s0896-6273(02)00942-x. [DOI] [PubMed] [Google Scholar]
- Nawa H, Carnahan J, Gall C. BDNF protein measured by a novel enzyme immunoassay in normal brain and after seizure: partial disagreement with mRNA levels. Eur J Neurosci. 1995;7:1527–1535. doi: 10.1111/j.1460-9568.1995.tb01148.x. [DOI] [PubMed] [Google Scholar]
- O'Neill MJ, Bleakman D, Zimmerman DM, Nisenbaum ES. AMPA receptor potentiators for the treatment of CNS disorders. Curr Drug Targets CNS Neurol Disord. 2004;3:181–194. doi: 10.2174/1568007043337508. [DOI] [PubMed] [Google Scholar]
- Park DC, Lautenschlager G, Hedden T, Davidson NS, Smith AD, Smith PK. Models of visuospatial and verbal memory across the adult life span. Psychol Aging. 2002;17:299–320. [PubMed] [Google Scholar]
- Patterson SL, Abel T, Deuel TAS, Martin KC, Rose JC, Kandel ER. Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron. 1996;16:1137–1145. doi: 10.1016/s0896-6273(00)80140-3. [DOI] [PubMed] [Google Scholar]
- Rex CS, Kramar EA, Colgin LL, Lin B, Gall CM, Lynch G. Long-term potentiation is impaired in middle-aged rats: regional specificity and reversal by adenosine receptor antagonists. J Neurosci. 2005;25:5956–5966. doi: 10.1523/JNEUROSCI.0880-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staubli U, Perez Y, Xu F, Rogers G, Ingvar M, Stone-Elander S, Lynch G. Centrally active modulators of glutamate (AMPA) receptors facilitate the induction of LTP in vivo. Proc Natl Acad Sci USA. 1994a;91:11158–11162. doi: 10.1073/pnas.91.23.11158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staubli U, Rogers G, Lynch G. Facilitation of glutamate receptors enhances memory. Proc Natl Acad Sci USA. 1994b;91:777–781. doi: 10.1073/pnas.91.2.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen EC, Mobley WC. Therapeutic potential of neurotrophic factors for neurological disorders. Ann Neurol. 1996;40:346–354. doi: 10.1002/ana.410400304. [DOI] [PubMed] [Google Scholar]


