Abstract
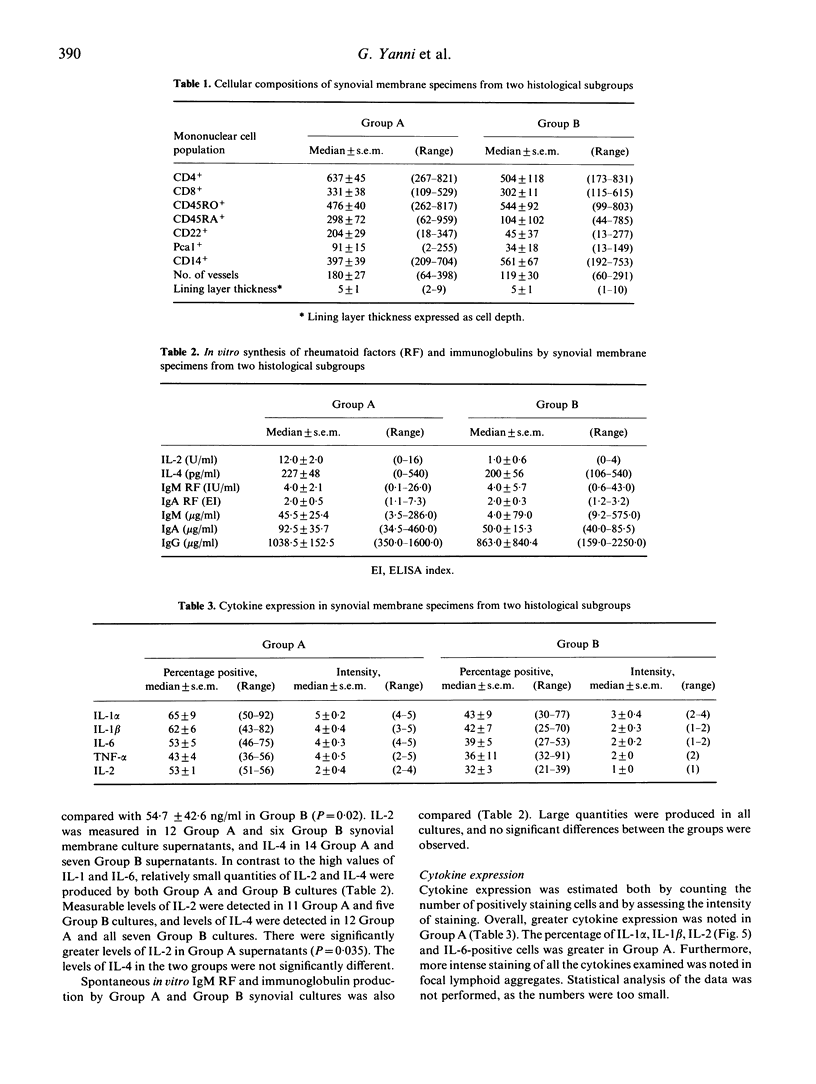
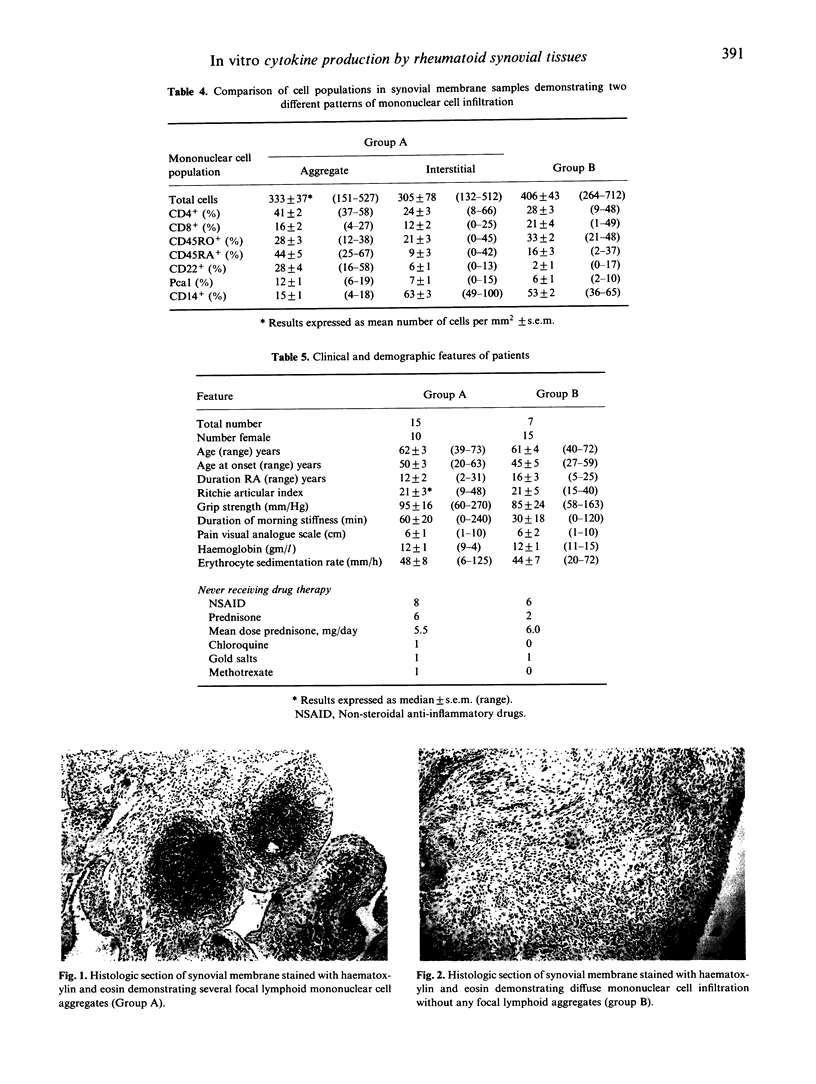
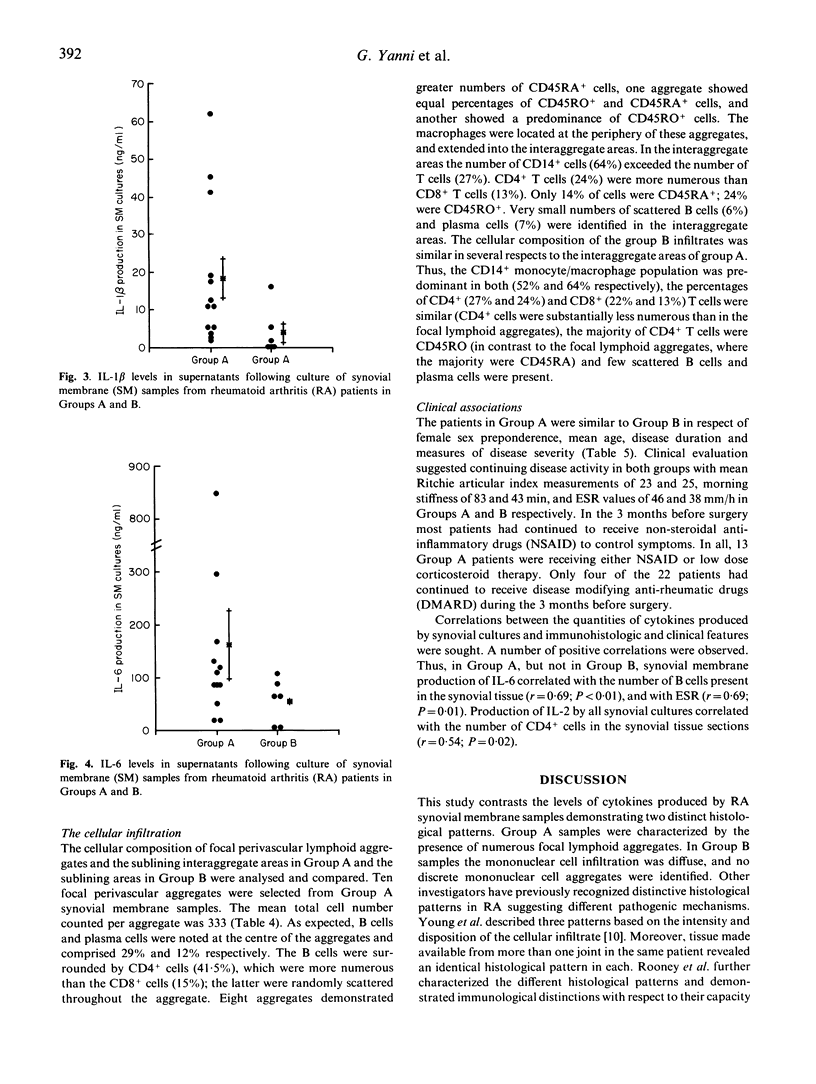
Synovial membrane samples obtained at knee arthroplasty from 22 patients with rheumatoid arthritis (RA) were characterized histologically. Two groups were identified. Tissue samples from 15 patients demonstrated multiple focal lymphoid aggregates of mononuclear cells (group A). Samples from the remaining seven patients demonstrated diffuse mononuclear cell infiltration (group B). Samples of each synovial membrane (0.25 g) were cultured for cytokine production. The highest levels of IL-1 beta and IL-6 were produced by group A tissues: 19.1 +/- 19.6 ng/ml IL-1 beta (mean +/- s.d.) and 264.4 +/- 301.9 ng/ml IL-6, versus 3.8 +/- 6.6 ng/ml and 54.7 +/- 42.6 ng/ml respectively. Small quantities of IL-2 and IL-4 were measured in both groups: the levels of IL-2 in group A cultures were highest (P = 0.04). Moreover, using MoAbs, the most intense cytokine staining in the tissues was detected in group A. Similar total numbers of each cell subpopulation and similar quantities of immunoglobulin and rheumatoid factor synthesis were measured in both groups. It is suggested that the presence of multiple focal lymphoid aggregates associated with higher levels of cytokine production observed in group A represent a greater degree of immunological activation, and may represent a subgroup of patients with a greater potential for articular destruction.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aarden L. A., De Groot E. R., Schaap O. L., Lansdorp P. M. Production of hybridoma growth factor by human monocytes. Eur J Immunol. 1987 Oct;17(10):1411–1416. doi: 10.1002/eji.1830171004. [DOI] [PubMed] [Google Scholar]
- Aarden L. A., De Groot E. R., Schaap O. L., Lansdorp P. M. Production of hybridoma growth factor by human monocytes. Eur J Immunol. 1987 Oct;17(10):1411–1416. doi: 10.1002/eji.1830171004. [DOI] [PubMed] [Google Scholar]
- Arend W. P., Dayer J. M. Cytokines and cytokine inhibitors or antagonists in rheumatoid arthritis. Arthritis Rheum. 1990 Mar;33(3):305–315. doi: 10.1002/art.1780330302. [DOI] [PubMed] [Google Scholar]
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Astaldi G. C., Janssen M. C., Lansdorp P., Willems C., Zeijlemaker W. P., Oosterhof F. Human endothelial culture supernatant (HECS): a growth factor for hybridomas. J Immunol. 1980 Oct;125(4):1411–1414. [PubMed] [Google Scholar]
- Bhardwaj N., Santhanam U., Lau L. L., Tatter S. B., Ghrayeb J., Rivelis M., Steinman R. M., Sehgal P. B., May L. T. IL-6/IFN-beta 2 in synovial effusions of patients with rheumatoid arthritis and other arthritides. Identification of several isoforms and studies of cellular sources. J Immunol. 1989 Oct 1;143(7):2153–2159. [PubMed] [Google Scholar]
- Boraschi D., Volpini G., Villa L., Nencioni L., Scapigliati G., Nucci D., Antoni G., Matteucci G., Cioli F., Tagliabue A. A monoclonal antibody to the IL-1 beta peptide 163-171 blocks adjuvanticity but not pyrogenicity of IL-1 beta in vivo. J Immunol. 1989 Jul 1;143(1):131–134. [PubMed] [Google Scholar]
- Buchan G., Barrett K., Turner M., Chantry D., Maini R. N., Feldmann M. Interleukin-1 and tumour necrosis factor mRNA expression in rheumatoid arthritis: prolonged production of IL-1 alpha. Clin Exp Immunol. 1988 Sep;73(3):449–455. [PMC free article] [PubMed] [Google Scholar]
- Cavender D., Haskard D., Yu C. L., Iguchi T., Miossec P., Oppenheimer-Marks N., Ziff M. Pathways to chronic inflammation in rheumatoid synovitis. Fed Proc. 1987 Jan;46(1):113–117. [PubMed] [Google Scholar]
- Chu C. Q., Field M., Feldmann M., Maini R. N. Localization of tumor necrosis factor alpha in synovial tissues and at the cartilage-pannus junction in patients with rheumatoid arthritis. Arthritis Rheum. 1991 Sep;34(9):1125–1132. doi: 10.1002/art.1780340908. [DOI] [PubMed] [Google Scholar]
- Combe B., Pope R. M., Fischbach M., Darnell B., Baron S., Talal N. Interleukin-2 in rheumatoid arthritis: production of and response to interleukin-2 in rheumatoid synovial fluid, synovial tissue and peripheral blood. Clin Exp Immunol. 1985 Mar;59(3):520–528. [PMC free article] [PubMed] [Google Scholar]
- Dayer J. M., Demczuk S. Cytokines and other mediators in rheumatoid arthritis. Springer Semin Immunopathol. 1984;7(4):387–413. doi: 10.1007/BF00201968. [DOI] [PubMed] [Google Scholar]
- Dayer J. M., de Rochemonteix B., Burrus B., Demczuk S., Dinarello C. A. Human recombinant interleukin 1 stimulates collagenase and prostaglandin E2 production by human synovial cells. J Clin Invest. 1986 Feb;77(2):645–648. doi: 10.1172/JCI112350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1 and its biologically related cytokines. Adv Immunol. 1989;44:153–205. doi: 10.1016/s0065-2776(08)60642-2. [DOI] [PubMed] [Google Scholar]
- Durum S. K., Schmidt J. A., Oppenheim J. J. Interleukin 1: an immunological perspective. Annu Rev Immunol. 1985;3:263–287. doi: 10.1146/annurev.iy.03.040185.001403. [DOI] [PubMed] [Google Scholar]
- Eastgate J. A., Symons J. A., Wood N. C., Grinlinton F. M., di Giovine F. S., Duff G. W. Correlation of plasma interleukin 1 levels with disease activity in rheumatoid arthritis. Lancet. 1988 Sep 24;2(8613):706–709. doi: 10.1016/s0140-6736(88)90185-7. [DOI] [PubMed] [Google Scholar]
- Faith A., Pontesilli O., Unger A., Panayi G. S., Johns P. ELISA assays for IgM and IgG rheumatoid factors. J Immunol Methods. 1982 Dec 17;55(2):169–177. doi: 10.1016/0022-1759(82)90029-1. [DOI] [PubMed] [Google Scholar]
- Firestein G. S., Alvaro-Gracia J. M., Maki R., Alvaro-Garcia J. M. Quantitative analysis of cytokine gene expression in rheumatoid arthritis. J Immunol. 1990 May 1;144(9):3347–3353. [PubMed] [Google Scholar]
- Fontana A., Hengartner H., Weber E., Fehr K., Grob P. J., Cohen G. Interleukin 1 activity in the synovial fluid of patients with rheumatoid arthritis. Rheumatol Int. 1982;2(2):49–53. doi: 10.1007/BF00541245. [DOI] [PubMed] [Google Scholar]
- Freemont A. J., Jones C. J., Bromley M., Andrews P. Changes in vascular endothelium related to lymphocyte collections in diseased synovia. Arthritis Rheum. 1983 Dec;26(12):1427–1433. doi: 10.1002/art.1780261203. [DOI] [PubMed] [Google Scholar]
- Gauldie J., Richards C., Harnish D., Lansdorp P., Baumann H. Interferon beta 2/B-cell stimulatory factor type 2 shares identity with monocyte-derived hepatocyte-stimulating factor and regulates the major acute phase protein response in liver cells. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7251–7255. doi: 10.1073/pnas.84.20.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Gowen M., Wood D. D., Ihrie E. J., Meats J. E., Russell R. G. Stimulation by human interleukin 1 of cartilage breakdown and production of collagenase and proteoglycanase by human chondrocytes but not by human osteoblasts in vitro. Biochim Biophys Acta. 1984 Feb 14;797(2):186–193. doi: 10.1016/0304-4165(84)90121-1. [DOI] [PubMed] [Google Scholar]
- Hamerman D., Wood D. D. Interleukin 1 enhances synovial cell hyaluronate synthesis. Proc Soc Exp Biol Med. 1984 Oct;177(1):205–210. doi: 10.3181/00379727-177-1-rc1. [DOI] [PubMed] [Google Scholar]
- Henderson B., Pettipher E. R. The synovial lining cell: biology and pathobiology. Semin Arthritis Rheum. 1985 Aug;15(1):1–32. doi: 10.1016/0049-0172(85)90007-1. [DOI] [PubMed] [Google Scholar]
- Hirano T., Matsuda T., Hosoi K., Okano A., Matsui H., Kishimoto T. Absence of antiviral activity in recombinant B cell stimulatory factor 2 (BSF-2). Immunol Lett. 1988 Jan;17(1):41–45. doi: 10.1016/0165-2478(88)90099-5. [DOI] [PubMed] [Google Scholar]
- Hirano T., Matsuda T., Turner M., Miyasaka N., Buchan G., Tang B., Sato K., Shimizu M., Maini R., Feldmann M. Excessive production of interleukin 6/B cell stimulatory factor-2 in rheumatoid arthritis. Eur J Immunol. 1988 Nov;18(11):1797–1801. doi: 10.1002/eji.1830181122. [DOI] [PubMed] [Google Scholar]
- Horii Y., Muraguchi A., Suematsu S., Matsuda T., Yoshizaki K., Hirano T., Kishimoto T. Regulation of BSF-2/IL-6 production by human mononuclear cells. Macrophage-dependent synthesis of BSF-2/IL-6 by T cells. J Immunol. 1988 Sep 1;141(5):1529–1535. [PubMed] [Google Scholar]
- Houssiau F. A., Devogelaer J. P., Van Damme J., de Deuxchaisnes C. N., Van Snick J. Interleukin-6 in synovial fluid and serum of patients with rheumatoid arthritis and other inflammatory arthritides. Arthritis Rheum. 1988 Jun;31(6):784–788. doi: 10.1002/art.1780310614. [DOI] [PubMed] [Google Scholar]
- Husby G., Williams R. C., Jr Immunohistochemical studies of interleukin-2 and gamma-interferon in rheumatoid arthritis. Arthritis Rheum. 1985 Feb;28(2):174–181. doi: 10.1002/art.1780280212. [DOI] [PubMed] [Google Scholar]
- Iguchi T., Kurosaka M., Ziff M. Electron microscopic study of HLA-DR and monocyte/macrophage staining cells in the rheumatoid synovial membrane. Arthritis Rheum. 1986 May;29(5):600–613. doi: 10.1002/art.1780290504. [DOI] [PubMed] [Google Scholar]
- Ishikawa H., Ziff M. Electron microscopic observations of immunoreactive cells in the rheumatoid synovial membrane. Arthritis Rheum. 1976 Jan-Feb;19(1):1–14. doi: 10.1002/art.1780190101. [DOI] [PubMed] [Google Scholar]
- Jirik F. R., Podor T. J., Hirano T., Kishimoto T., Loskutoff D. J., Carson D. A., Lotz M. Bacterial lipopolysaccharide and inflammatory mediators augment IL-6 secretion by human endothelial cells. J Immunol. 1989 Jan 1;142(1):144–147. [PubMed] [Google Scholar]
- Kohase M., Henriksen-DeStefano D., May L. T., Vilcek J., Sehgal P. B. Induction of beta 2-interferon by tumor necrosis factor: a homeostatic mechanism in the control of cell proliferation. Cell. 1986 Jun 6;45(5):659–666. doi: 10.1016/0092-8674(86)90780-4. [DOI] [PubMed] [Google Scholar]
- Krane S. M., Dayer J. M., Simon L. S., Byrne M. S. Mononuclear cell-conditioned medium containing mononuclear cell factor (MCF), homologous with interleukin 1, stimulates collagen and fibronectin synthesis by adherent rheumatoid synovial cells: effects of prostaglandin E2 and indomethacin. Coll Relat Res. 1985 Mar;5(2):99–117. doi: 10.1016/s0174-173x(85)80033-9. [DOI] [PubMed] [Google Scholar]
- Kurosaka M., Ziff M. Immunoelectron microscopic study of the distribution of T cell subsets in rheumatoid synovium. J Exp Med. 1983 Oct 1;158(4):1191–1210. doi: 10.1084/jem.158.4.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalor P. A., Mapp P. I., Hall P. A., Revell P. A. Proliferative activity of cells in the synovium as demonstrated by a monoclonal antibody, Ki67. Rheumatol Int. 1987;7(5):183–186. doi: 10.1007/BF00541375. [DOI] [PubMed] [Google Scholar]
- Lotz M., Jirik F., Kabouridis P., Tsoukas C., Hirano T., Kishimoto T., Carson D. A. B cell stimulating factor 2/interleukin 6 is a costimulant for human thymocytes and T lymphocytes. J Exp Med. 1988 Mar 1;167(3):1253–1258. doi: 10.1084/jem.167.3.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone D. G., Wahl S. M., Tsokos M., Cattell H., Decker J. L., Wilder R. L. Immune function in severe, active rheumatoid arthritis. A relationship between peripheral blood mononuclear cell proliferation to soluble antigens and synovial tissue immunohistologic characteristics. J Clin Invest. 1984 Oct;74(4):1173–1185. doi: 10.1172/JCI111526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mapp P. I., Revell P. A. Ultrastructural characterisation of macrophages (type A cells) in the synovial lining. Rheumatol Int. 1988;8(4):171–176. doi: 10.1007/BF00270456. [DOI] [PubMed] [Google Scholar]
- Mason D. Y., Cordell J. L., Abdulaziz Z., Naiem M., Bordenave G. Preparation of peroxidase: antiperoxidase (PAP) complexes for immunohistological labeling of monoclonal antibodies. J Histochem Cytochem. 1982 Nov;30(11):1114–1122. doi: 10.1177/30.11.6183312. [DOI] [PubMed] [Google Scholar]
- Meijer C. J., de Graaff-Reitsma C. B., Lafeber G. J., Cats A. In situ localization of lymphocyte subsets in synovial membranes of patients with rheumatoid arthritis with monoclonal antibodies. J Rheumatol. 1982 May-Jun;9(3):359–365. [PubMed] [Google Scholar]
- Miossec P., Dinarello C. A., Ziff M. Interleukin-1 lymphocyte chemotactic activity in rheumatoid arthritis synovial fluid. Arthritis Rheum. 1986 Apr;29(4):461–470. doi: 10.1002/art.1780290402. [DOI] [PubMed] [Google Scholar]
- Miossec P., Naviliat M., Dupuy d'Angeac A., Sany J., Banchereau J. Low levels of interleukin-4 and high levels of transforming growth factor beta in rheumatoid synovitis. Arthritis Rheum. 1990 Aug;33(8):1180–1187. doi: 10.1002/art.1780330819. [DOI] [PubMed] [Google Scholar]
- Miyasaka N., Sato K., Goto M., Sasano M., Natsuyama M., Inoue K., Nishioka K. Augmented interleukin-1 production and HLA-DR expression in the synovium of rheumatoid arthritis patients. Possible involvement in joint destruction. Arthritis Rheum. 1988 Apr;31(4):480–486. doi: 10.1002/art.1780310404. [DOI] [PubMed] [Google Scholar]
- Miyasaka N., Sato K., Hashimoto J., Kohsaka H., Yamamoto K., Goto M., Inoue K., Matsuda T., Hirano T., Kishimoto T. Constitutive production of interleukin 6/B cell stimulatory factor-2 from inflammatory synovium. Clin Immunol Immunopathol. 1989 Aug;52(2):238–247. doi: 10.1016/0090-1229(89)90175-x. [DOI] [PubMed] [Google Scholar]
- Noma T., Mizuta T., Rosén A., Hirano T., Kishimoto T., Honjo T. Enhancement of the interleukin 2 receptor expression on T cells by multiple B-lymphotropic lymphokines. Immunol Lett. 1987 Jul;15(3):249–253. doi: 10.1016/0165-2478(87)90032-0. [DOI] [PubMed] [Google Scholar]
- Nouri A. M., Panayi G. S., Goodman S. M. Cytokines and the chronic inflammation of rheumatic disease. I. The presence of interleukin-1 in synovial fluids. Clin Exp Immunol. 1984 Feb;55(2):295–302. [PMC free article] [PubMed] [Google Scholar]
- Paul W. E., Ohara J. B-cell stimulatory factor-1/interleukin 4. Annu Rev Immunol. 1987;5:429–459. doi: 10.1146/annurev.iy.05.040187.002241. [DOI] [PubMed] [Google Scholar]
- Pettipher E. R., Higgs G. A., Henderson B. Interleukin 1 induces leukocyte infiltration and cartilage proteoglycan degradation in the synovial joint. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8749–8753. doi: 10.1073/pnas.83.22.8749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie D. M., Boyle J. A., McInnes J. M., Jasani M. K., Dalakos T. G., Grieveson P., Buchanan W. W. Clinical studies with an articular index for the assessment of joint tenderness in patients with rheumatoid arthritis. Q J Med. 1968 Jul;37(147):393–406. [PubMed] [Google Scholar]
- Rooney M., Condell D., Quinlan W., Daly L., Whelan A., Feighery C., Bresnihan B. Analysis of the histologic variation of synovitis in rheumatoid arthritis. Arthritis Rheum. 1988 Aug;31(8):956–963. doi: 10.1002/art.1780310803. [DOI] [PubMed] [Google Scholar]
- Rooney M., Whelan A., Feighery C., Bresnihan B. The immunohistologic features of synovitis, disease activity and in vitro IgM rheumatoid factor synthesis by blood mononuclear cells in rheumatoid arthritis. J Rheumatol. 1989 Apr;16(4):459–467. [PubMed] [Google Scholar]
- Smith K. A., Favata M. F., Oroszlan S. Production and characterization of monoclonal antibodies to human interleukin 2: strategy and tactics. J Immunol. 1983 Oct;131(4):1808–1815. [PubMed] [Google Scholar]
- Takai Y., Wong G. G., Clark S. C., Burakoff S. J., Herrmann S. H. B cell stimulatory factor-2 is involved in the differentiation of cytotoxic T lymphocytes. J Immunol. 1988 Jan 15;140(2):508–512. [PubMed] [Google Scholar]
- Taniguchi T., Matsui H., Fujita T., Hatakeyama M., Kashima N., Fuse A., Hamuro J., Nishi-Takaoka C., Yamada G. Molecular analysis of the interleukin-2 system. Immunol Rev. 1986 Aug;92:121–133. doi: 10.1111/j.1600-065x.1986.tb01497.x. [DOI] [PubMed] [Google Scholar]
- Thorpe R., Wadhwa M., Gearing A., Mahon B., Poole S. Sensitive and specific immunoradiometric assays for human interleukin-1 alpha. Lymphokine Res. 1988 Summer;7(2):119–127. [PubMed] [Google Scholar]
- Tosato G., Pike S. E. Interferon-beta 2/interleukin 6 is a co-stimulant for human T lymphocytes. J Immunol. 1988 Sep 1;141(5):1556–1562. [PubMed] [Google Scholar]
- Van Damme J., Cayphas S., Opdenakker G., Billiau A., Van Snick J. Interleukin 1 and poly(rI).poly(rC) induce production of a hybridoma growth factor by human fibroblasts. Eur J Immunol. 1987 Jan;17(1):1–7. doi: 10.1002/eji.1830170102. [DOI] [PubMed] [Google Scholar]
- Waage A., Kaufmann C., Espevik T., Husby G. Interleukin-6 in synovial fluid from patients with arthritis. Clin Immunol Immunopathol. 1989 Mar;50(3):394–398. doi: 10.1016/0090-1229(89)90146-3. [DOI] [PubMed] [Google Scholar]
- Wood D. D., Ihrie E. J., Dinarello C. A., Cohen P. L. Isolation of an interleukin-1-like factor from human joint effusions. Arthritis Rheum. 1983 Aug;26(8):975–983. doi: 10.1002/art.1780260806. [DOI] [PubMed] [Google Scholar]
- Wood N. C., Dickens E., Symons J. A., Duff G. W. In situ hybridization of interleukin-1 in CD14-positive cells in rheumatoid arthritis. Clin Immunol Immunopathol. 1992 Mar;62(3):295–300. doi: 10.1016/0090-1229(92)90106-x. [DOI] [PubMed] [Google Scholar]
- Wood N. C., Symons J. A., Dickens E., Duff G. W. In situ hybridization of IL-6 in rheumatoid arthritis. Clin Exp Immunol. 1992 Feb;87(2):183–189. doi: 10.1111/j.1365-2249.1992.tb02972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young C. L., Adamson T. C., 3rd, Vaughan J. H., Fox R. I. Immunohistologic characterization of synovial membrane lymphocytes in rheumatoid arthritis. Arthritis Rheum. 1984 Jan;27(1):32–39. doi: 10.1002/art.1780270106. [DOI] [PubMed] [Google Scholar]





