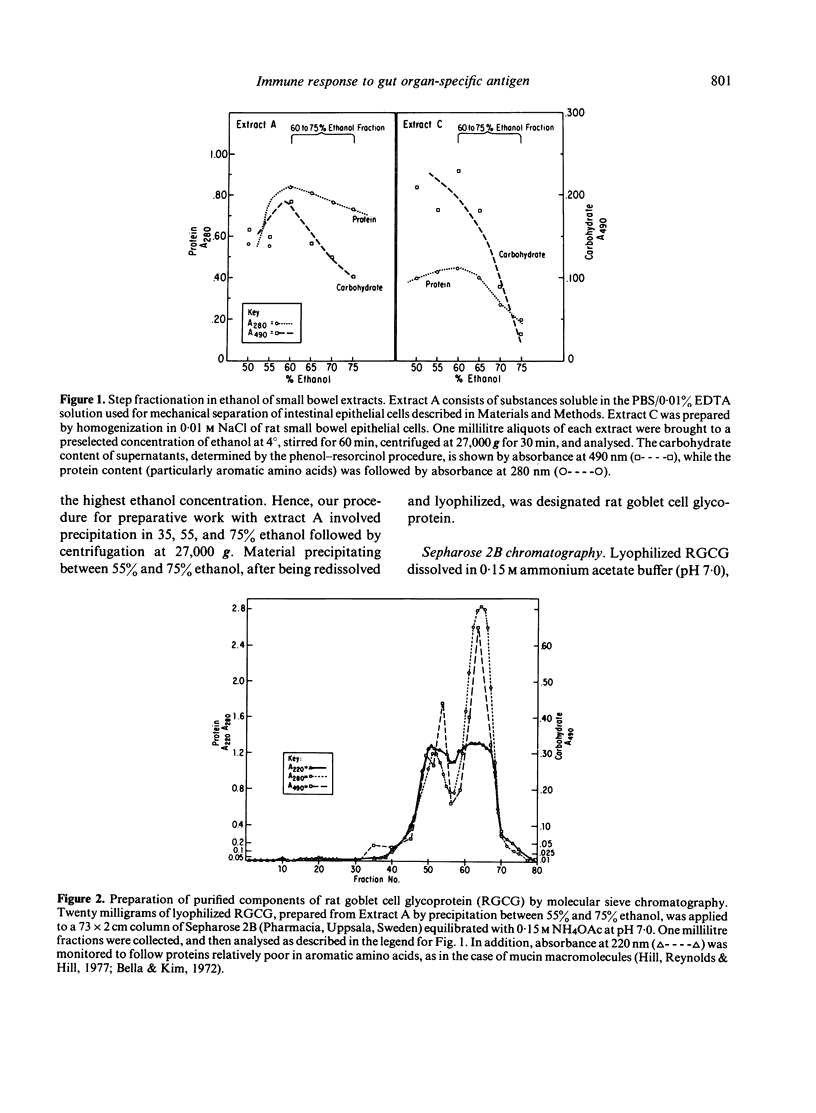
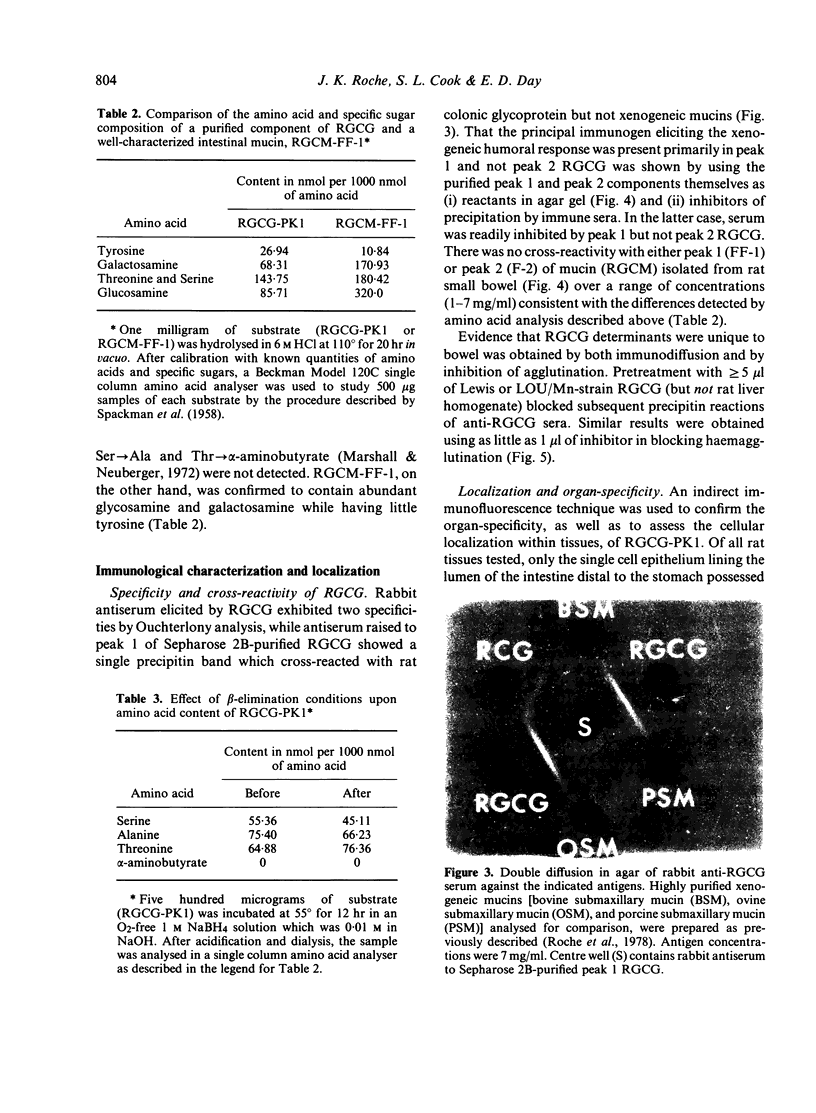
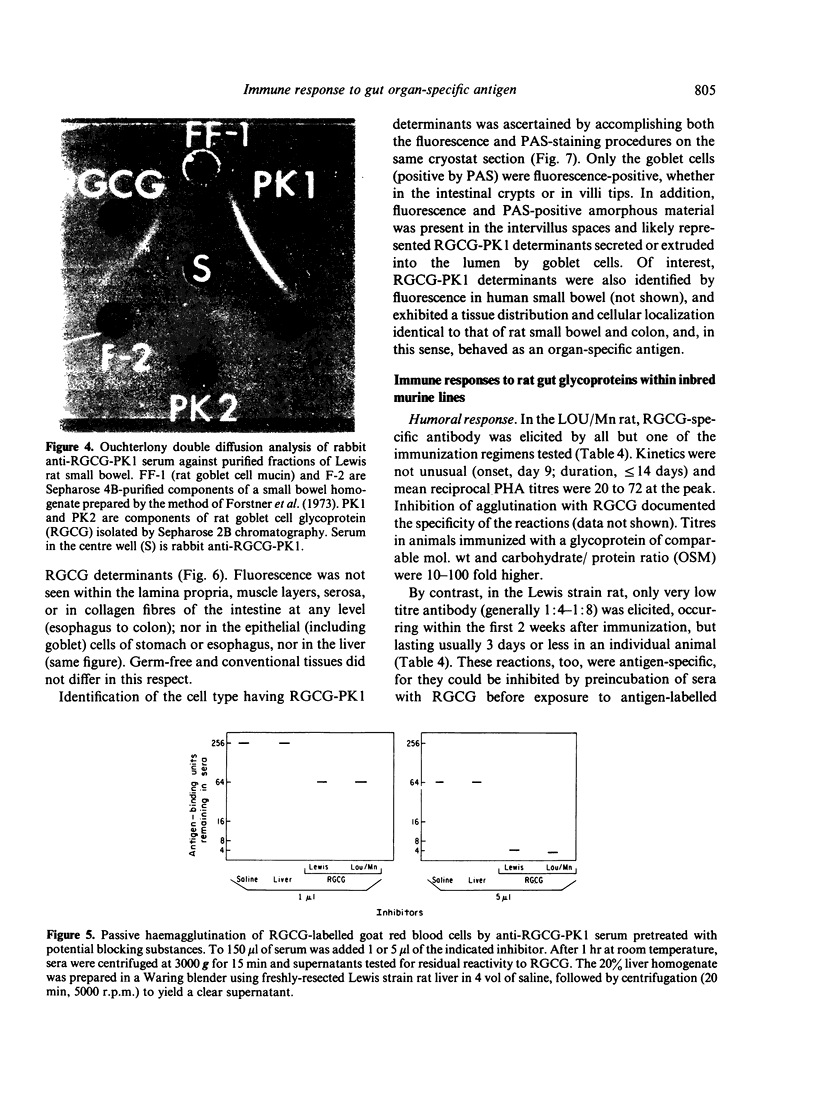
Abstract
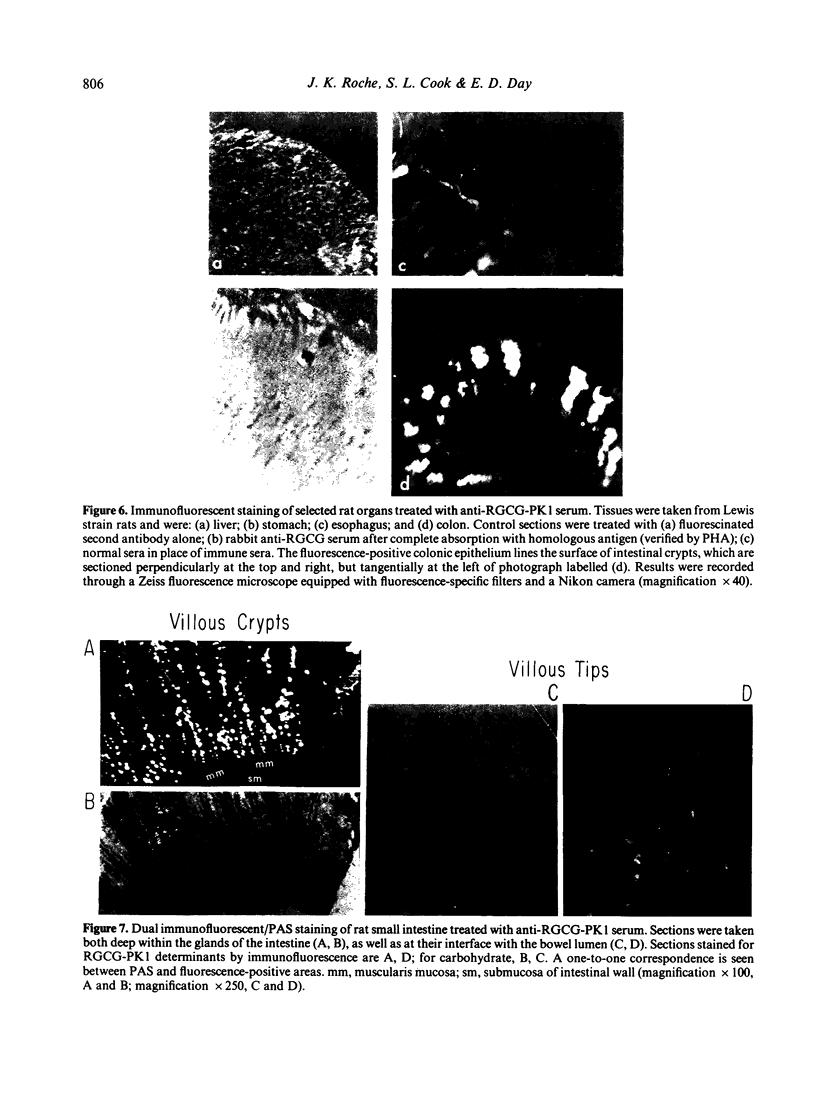
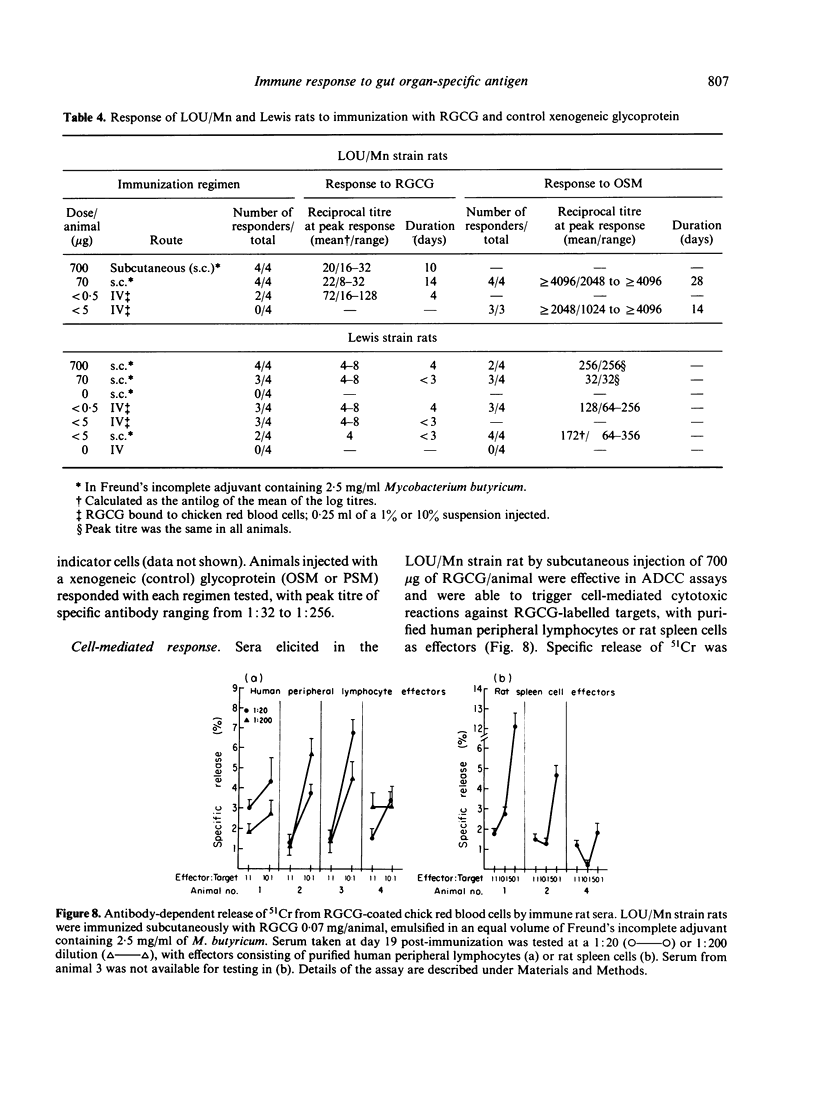
We report, for the first time, immune responses within two lines of inbred rats to a purified Lewis rat glycoprotein antigen which is organ-specific for intestine. The antigen was prepared by solubilization of gut epithelial cell-associated macromolecules, fractionation in ethanol, and molecular sieve chromatography over Sepharose 2B. Homogeneity of the end product (RGCG-PK1) was supported by results of both double diffusion in agar and SDS polyacrylamide gel electrophoresis. Amino acid analysis and specific sugar determination proved that RGCG-PK1 was not a classical mucin because of its comparatively high tyrosine and low galactosamine + glucosamine content, and the absence of glycosidic linkages to serine and threonine. Organ-specificity was shown by the ability of RGCG (but not liver homogenate) to inhibit precipitation and haemagglutination by heterologous specific sera. Organ-specificity was confirmed by the demonstration of RGCG-PK1-specific immunofluorescence staining of rat small and large intestine, but not esophagus, stomach or liver. RGCG-PK1 determinants within rat and human small bowel were found to be confined to goblet cells and intestinal glycocalyx. Anti-RCGC-PK1 serum showed no reactivity with highly purified xenogeneic mucins nor with syngeneic small bowel mucin. Specific antibody (as well as antibody-dependent cellular cytotoxicity) to RGCG was elicited and detected for up to 10 days in two lines of inbred rats, including the one (Lewis) from which the antigen was isolated. The duration and peak of the humoral immune response were abbreviated compared with that of a xenogeneic control glycoprotein studied in parallel, probably due to immunoregulatory mechanisms operative for self antigens.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bella A., Jr, Kim Y. S. Rat small intestinal mucin: isolation and characterization of a water-soluble mucin fraction. Arch Biochem Biophys. 1972 Jun;150(2):679–689. doi: 10.1016/0003-9861(72)90086-0. [DOI] [PubMed] [Google Scholar]
- Bøyum A. Isolation of lymphocytes, granulocytes and macrophages. Scand J Immunol. 1976 Jun;Suppl 5:9–15. [PubMed] [Google Scholar]
- Culling C. F., Reid P. E., Trueman L. S., Dunn W. L. A simple method for the isolation of viable epithelial cells of the gastrointestinal (GI) tract. Proc Soc Exp Biol Med. 1973 Feb;142(2):434–438. doi: 10.3181/00379727-142-37040. [DOI] [PubMed] [Google Scholar]
- FREUND J., LIPTON M. M., THOMPSON G. E. Aspermatogenesis in the guinea pig induced by testicular tissue and adjuvants. J Exp Med. 1953 May;97(5):711–726. doi: 10.1084/jem.97.5.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstner J., Taichman N., Kalnins V., Forstner G. Intestinal goblet cell mucus: isolation and identification by immunofluorescence of a goblet cell glycoprotein. J Cell Sci. 1973 Mar;12(2):585–602. doi: 10.1242/jcs.12.2.585. [DOI] [PubMed] [Google Scholar]
- HOLBOROW E. J., ASHERSON G. L., WIGLEY R. D. AUTOANTIBODY PRODUCTION IN RABBITS. VI. THE PRODUCTION OF AUTOANTIBODIES AGAINST RABBIT GASTRIC, ILEAL AND COLONIC MUCOSA. Immunology. 1963 Nov;6:551–560. [PMC free article] [PubMed] [Google Scholar]
- Hill H. D., Jr, Reynolds J. A., Hill R. L. Purification, composition, molecular weight, and subunit structure of ovine submaxillary mucin. J Biol Chem. 1977 Jun 10;252(11):3791–3798. [PubMed] [Google Scholar]
- Kopp W. L., Trier J. S., Mackenzie I. L., Donaldson R. M., Jr Antibodies to intestinal microvillous membranes. I. Characterization and morphologic localization. J Exp Med. 1968 Aug 1;128(2):357–373. doi: 10.1084/jem.128.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lagercrantz R., Hammarström S., Perlmann P., Gustafsson B. E. Immunological studies in ulcerative colitis. 3. Incidence of antibodies to colon-antigen in ulcerative colitis and other gastro-intestinal diseases. Clin Exp Immunol. 1966 Jul;1(3):263–276. [PMC free article] [PubMed] [Google Scholar]
- NAIRN R. C., FOTHERGILL J. E., McENTEGART M. G., PORTEOUS I. B. Gastro-intestinal-specific antigen: an immunohistological and serological study. Br Med J. 1962 Jun 30;1(5295):1788–1790. doi: 10.1136/bmj.1.5295.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura R. M., Weigle W. O. Transfer of experimental autoimmune thyroiditis by serum from thyroidectomized donors. J Exp Med. 1969 Aug 1;130(2):263–285. doi: 10.1084/jem.130.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATERSON P. Y. Transfer of allergic encephalomyelitis in rats by means of lymph node cells. J Exp Med. 1960 Jan 1;111:119–136. doi: 10.1084/jem.111.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin B. S. Duodenum-, ileum- and colon-specific antigens. Int Arch Allergy Appl Immunol. 1976;50(2):133–141. doi: 10.1159/000231490. [DOI] [PubMed] [Google Scholar]
- Roche J. K., Cook S. R., Day E. D. Cellular cytotoxicity and gastrointestinal inflammation in inbred rats: induction with gut organ-specific antigens. Immunology. 1981 Nov;44(3):489–497. [PMC free article] [PubMed] [Google Scholar]
- Roche J. K., Day E. D., Hill H. D. Rabbit antibodies to ovine-submaxillary mucin. Detection, specificity and cross-reactivity. Immunochemistry. 1978 May;15(5):339–343. doi: 10.1016/0161-5890(78)90096-2. [DOI] [PubMed] [Google Scholar]
- Roche J. K., Varitek V. A., Hill H. D., Day E. D. Specificity and T-lymphocyte dependence of the humoral immune response in the rat to purified ovine and porcine mucins. Mol Immunol. 1979 Aug;16(8):609–619. doi: 10.1016/0161-5890(79)90124-x. [DOI] [PubMed] [Google Scholar]
- Watson D. W., Quigley A., Bolt R. J. Effect of lymphocytes from patients with ulcerative colitis on human adult colon epithelial cells. Gastroenterology. 1966 Dec;51(6):985–993. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]