Abstract
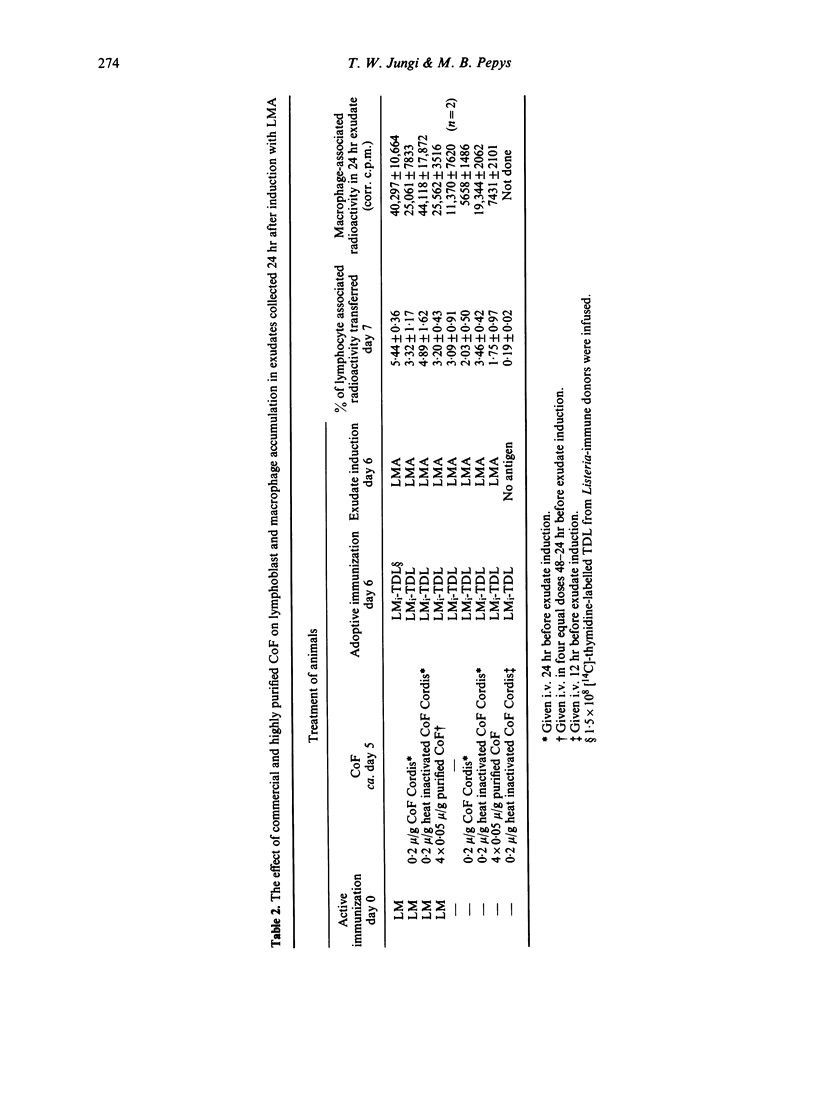
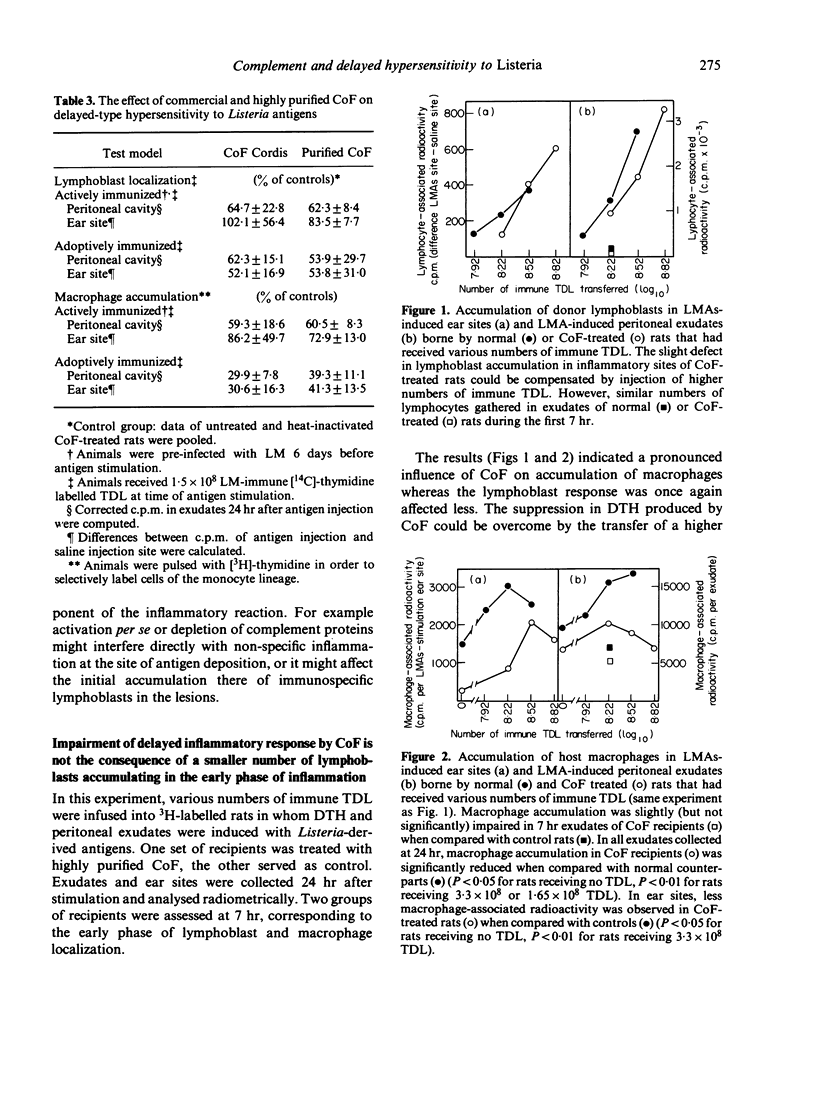
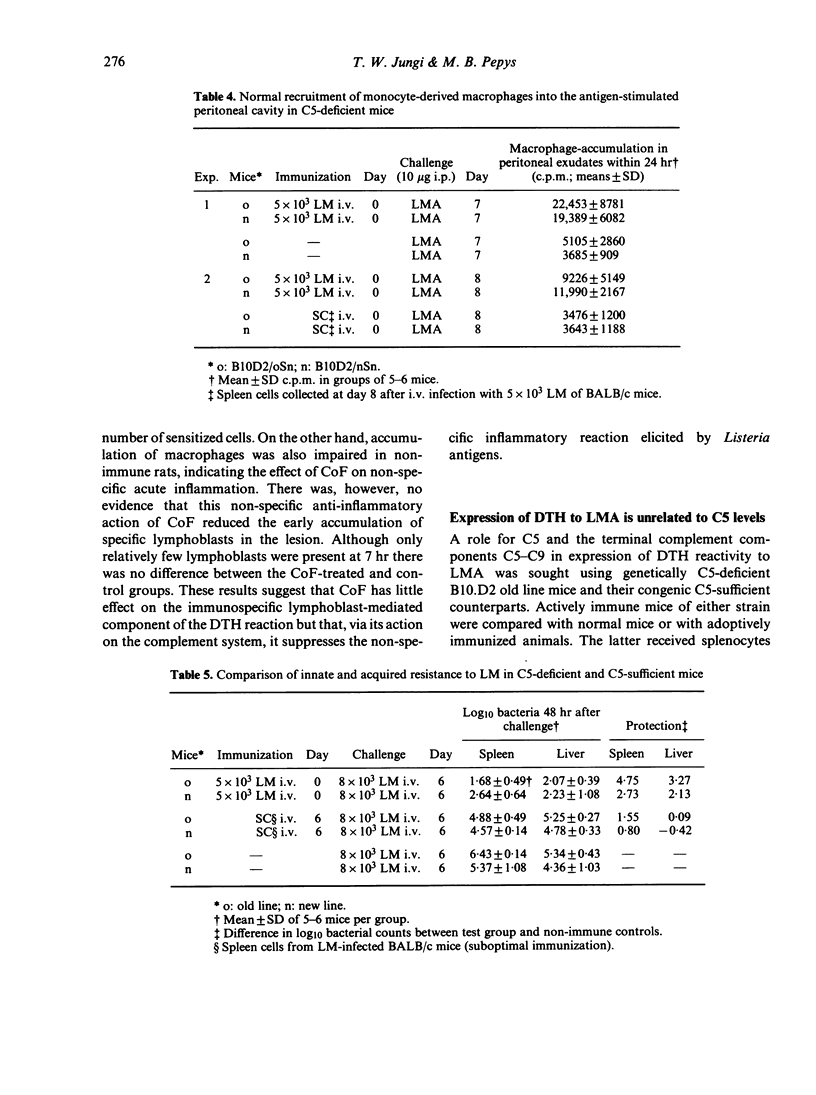
The in vivo effect of cobra factor (CoF), the complement-activating protein of cobra (Naja naja) venom, was investigated, using quantifiable assays for localization of labelled donor lymphoblasts and of host macrophages in intraperitoneal and subcutaneous sites of injection of antigens from Listeria monocytogenes. Both commercially available (Cordis) and highly purified CoF impaired these inflammatory responses, suggesting that the complement-activating protein was itself responsible rather than lymphocytotoxic or other contaminants. CoF had no measurable effect on lymphoblast localization during the first 7 hr, and only a slight effect at 24 hr, whereas macrophage accumulation was reduced by about 50% at 24 hr. This suggests that CoF treatment affected non-specific components of the early inflammatory reaction but had little or no effect on the subsequent immunospecific reaction. The effect of CoF on macrophages may be direct, or via depletion of complement components acting on macrophages, such as factor B and/or C3 or fragments thereof. It does not seem to involve the terminal complement components, C5--C9, since neither delayed-type hypersensitivity (DTH) nor cellular resistance to Listeria was reduced in C5-deficient mice when compared with C5-sufficient congenic controls.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballow M., Day N. K., Good R. A. Effect of cobra venom factor on the local GVH reaction. I. Partial characterization of a cytotoxic factor from cobra venom for rat lymphocytes. J Immunol. 1973 Feb;110(2):354–361. [PubMed] [Google Scholar]
- Bianco C., Götze O., Cohn Z. A. Regulation of macrophage migration by products of the complement system. Proc Natl Acad Sci U S A. 1979 Feb;76(2):888–891. doi: 10.1073/pnas.76.2.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheers C., Waller R. Activated macrophages in congenitally athymic "nude mice" and in lethally irradiate mice. J Immunol. 1975 Sep;115(3):844–847. [PubMed] [Google Scholar]
- Cochrane C. G., Müller-Eberhard H. J., Aikin B. S. Depletion of plasma complement in vivo by a protein of cobra venom: its effect on various immunologic reactions. J Immunol. 1970 Jul;105(1):55–69. [PubMed] [Google Scholar]
- Götze O., Bianco C., Cohn Z. A. The induction of macrophage spreading by factor B of the properdin system. J Exp Med. 1979 Feb 1;149(2):372–386. doi: 10.1084/jem.149.2.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill W. C. The influence of the cellular infiltrate on the evolution and intensity of delayed hypersensitivity reactions. J Exp Med. 1969 Feb 1;129(2):363–370. doi: 10.1084/jem.129.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungi T. W., McGregor D. D. Activated lymphocytes trigger lymphoblast extravasation. Cell Immunol. 1978 Jun;38(1):76–83. doi: 10.1016/0008-8749(78)90033-3. [DOI] [PubMed] [Google Scholar]
- Jungi T. W., McGregor D. D. Allogeneic restriction of acquired antimicrobial resistance in the rat. J Immunol. 1978 Aug;121(2):449–455. [PubMed] [Google Scholar]
- Jungi T. W., McGregor D. D. Generation of macrophage chemotactic activity in situ in Listeria-immune rats. Cell Immunol. 1977 Oct;33(2):322–339. doi: 10.1016/0008-8749(77)90162-9. [DOI] [PubMed] [Google Scholar]
- Jungi T. W., McGregor D. D. Role of complement in the expression of delayed-type hypersensitivity in rats: studies with cobra venom factor. Infect Immun. 1979 Mar;23(3):633–643. doi: 10.1128/iai.23.3.633-643.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostiala A. A., McGregor D. D. The mediator of cellular immunity. IX. The relationship between cellular hypersensitivity and acquired cellular resistance in rats infected with Listeria monocytogenes. J Exp Med. 1975 Jun 1;141(6):1249–1260. doi: 10.1084/jem.141.6.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachmann P. J., Halbwachs L., Gewurz A., Gewurz H. Purification of cobra venom factor from phospholipase A contaminant. Immunology. 1976 Dec;31(6):961–968. [PMC free article] [PubMed] [Google Scholar]
- Lawrence D. A., Schell R. F. Susceptibility of C5-deficient mice to listeriosis: modulation by Concanavalin A. Cell Immunol. 1978 Sep;39(2):336–344. doi: 10.1016/0008-8749(78)90109-0. [DOI] [PubMed] [Google Scholar]
- McGregor D. D., Logie P. S. The mediator of cellular immunity. VII. Localization of sensitized lymphocytes in inflammatory exudates. J Exp Med. 1974 Jun 1;139(6):1415–1430. doi: 10.1084/jem.139.6.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor D. D., Logie P. S. The mediator of cellular immunity. X. Interaction of macrophages and specifically sensitized lymphocytes. Cell Immunol. 1975 Aug;18(2):454–465. doi: 10.1016/0008-8749(75)90072-6. [DOI] [PubMed] [Google Scholar]
- Morrison D. C., Louis J. A., Weigle W. O. Dissociation of anticomplementary and adjuvant properties of proteins derived from cobra venom. Immunology. 1976 Mar;30(3):317–323. [PMC free article] [PubMed] [Google Scholar]
- Newborg M. F., North R. J. On the mechanism of T cell-independent anti-Listeria resistance in nude mice. J Immunol. 1980 Feb;124(2):571–576. [PubMed] [Google Scholar]
- Pepys M. B. Role of complement in induction of antibody production in vivo. Effect of cobra factor and other C3-reactive agents on thymus-dependent and thymus-independent antibody responses. J Exp Med. 1974 Jul 1;140(1):126–145. doi: 10.1084/jem.140.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepys M. B. Role of complement in induction of the allergic response. Nat New Biol. 1972 May 31;237(74):157–159. doi: 10.1038/newbio237157a0. [DOI] [PubMed] [Google Scholar]
- Pepys M. B. Role of complement in the induction of immunological responses. Transplant Rev. 1976;32:93–120. doi: 10.1111/j.1600-065x.1976.tb00230.x. [DOI] [PubMed] [Google Scholar]
- Pepys M. B. Studies in vivo of cobra factor and murine C3. Immunology. 1975 Feb;28(2):369–377. [PMC free article] [PubMed] [Google Scholar]
- Pepys M. B., Tompkins C., Smith A. D. An improved method for the isolation from Naja naja venom of cobra factor (CoF) free of phospholipase A. J Immunol Methods. 1979;30(2):105–117. doi: 10.1016/0022-1759(79)90085-1. [DOI] [PubMed] [Google Scholar]
- Rumjanek V. M., Brent L., Pepys M. B. Cell-mediated immunological responsiveness in mice decomplemented with cobra venom factor. Immunology. 1978 Jun;34(6):1117–1123. [PMC free article] [PubMed] [Google Scholar]
- Skamene E., Kongshavn P. A., Sachs D. H. Resistance to Listeria monocytogenes in mice: genetic control by genes that are not linked to the H-2 complex. J Infect Dis. 1979 Feb;139(2):228–231. doi: 10.1093/infdis/139.2.228. [DOI] [PubMed] [Google Scholar]
- Snyderman R., Phillips J. K., Mergenhagen S. E. Biological activity of complement in vivo. Role of C5 in the accumulation of polymorphonuclear leukocytes in inflammatory exudates. J Exp Med. 1971 Nov 1;134(5):1131–1143. doi: 10.1084/jem.134.5.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]


