Abstract
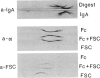
Human or rat purified secretory IgA (sIgA) injected intravenously (i.v.) into rats is transferred to bile much less (seven to twenty-four times) than human or rat polymeric IgA (pIgA) devoid of secretory component (SC). A polymeric Fc alpha (pFc alpha) fragment of a human IgAl myeloma protein, obtained by IgA-protease digestion, bound in vitro to rat SC and was actively transferred in vivo into bile, in contrast to the corresponding Fab alpha. The IgA recovered in bile was not degraded, as judged by sedimentation in density gradients. Purified rabbit IgG anti-rat SC antibody was also efficiently transported in vivo into bile, about forty times more than normal rabbit IgG. The biliary transport of anti-Sc antibody could be reduced and retarded by the simultaneous i.v. injection of purified rat SC or human pIgA. The transfer of rat 125I-pIgA into bile was also significantly reduced and retarded by the concomitant i.v. injection of purified rat or human SC. Moreover, i.v. injection of purified rat or human SC induced a marked and prolonged decrease of the sIgA level in the bile. Rat SC was more effective than human SC in this respect. All these in vivo experiments confirm the in vitro findings of Orlans, Peppard, Fry, Hinton : Mullock, (1979) and Socken, Jeejeebhoy, Bazin & Underdown, (1979) showing that Sc is the IgA-receptor on the hepatocyte membrane for the transfer of pIgA from rat plasma into bile.
Full text
PDF


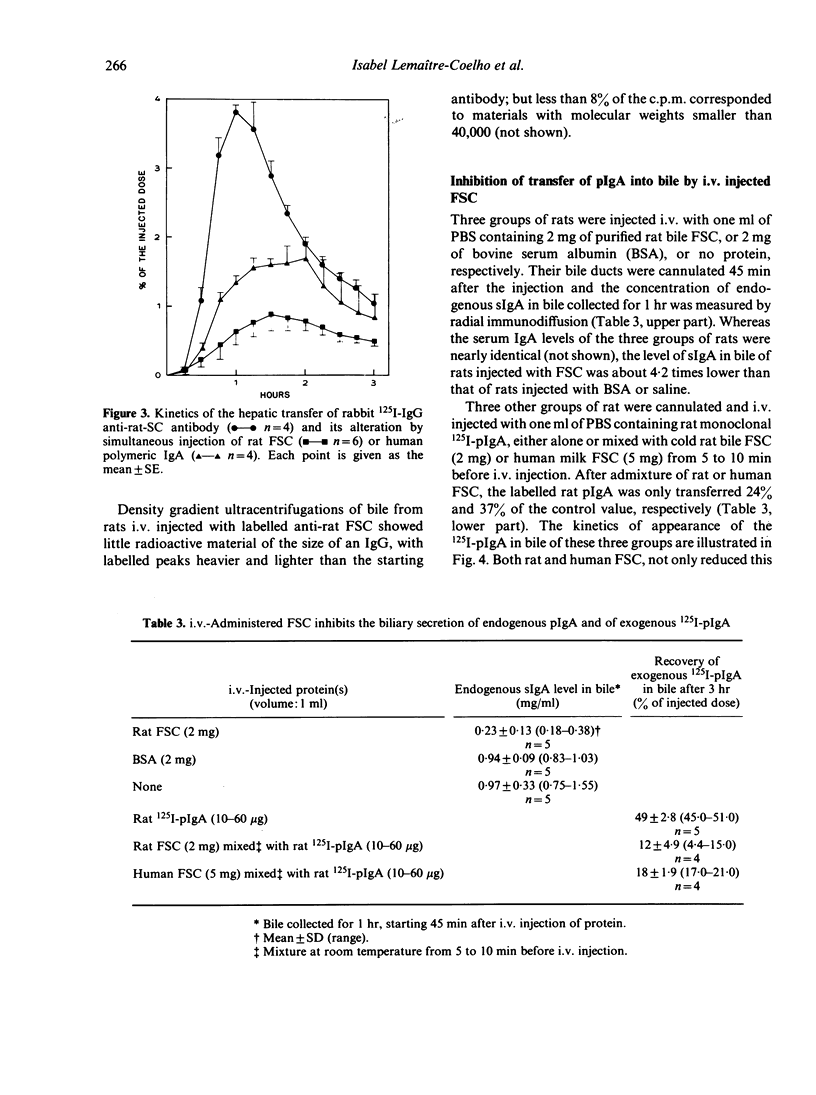
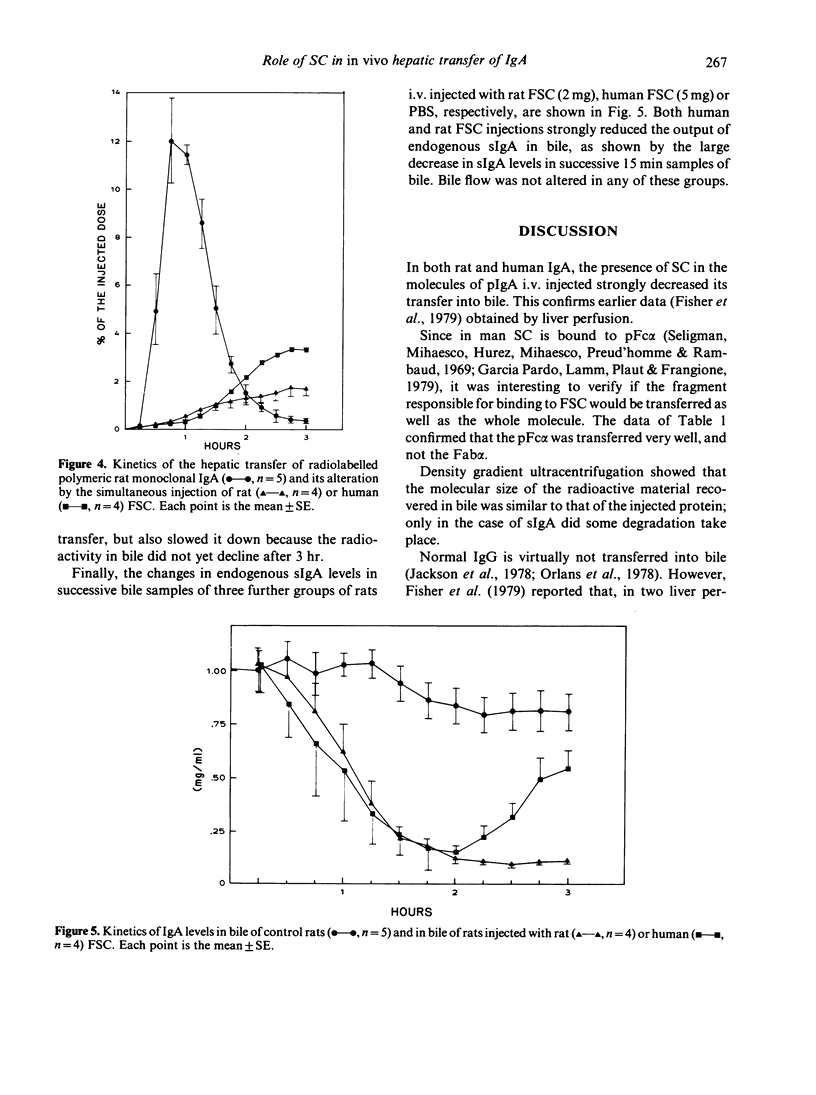



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altamirano G. A., Barranco-Acosta C., van Roost E., Vaerman J. P. Isolation and characterization of secretory IgA (sIgA) and free secretory component (FSC) from rat bile. Mol Immunol. 1980 Dec;17(12):1525–1537. doi: 10.1016/0161-5890(80)90178-9. [DOI] [PubMed] [Google Scholar]
- Birbeck M. S., Cartwright P., Hall J. G., Orlans E., Peppard J. The transport by hepatocytes of immunoglobulin A from blood to bile visualized by autoradiography and electron microscopy. Immunology. 1979 Jun;37(2):477–484. [PMC free article] [PubMed] [Google Scholar]
- Brandtzaeg P. Mucosal and glandular distribution of immunoglobulin components: differential localization of free and bound SC in secretory epithelial cells. J Immunol. 1974 Apr;112(4):1553–1559. [PubMed] [Google Scholar]
- Brandtzaeg P. Polymeric IgA is complexed with secretory component (SC) on the surface of human intestinal epithelial cells. Scand J Immunol. 1978;8(1):39–52. doi: 10.1111/j.1365-3083.1978.tb00494.x. [DOI] [PubMed] [Google Scholar]
- Cambiaso C. L., Goffinet A., Vaerman J. P., Heremans J. F. Glutaraldehyde-activated aminohexyl- derivative of Sepharose 4B as a new verstile immunoabsorbent. Immunochemistry. 1975 Apr;12(4):273–278. doi: 10.1016/0019-2791(75)90175-5. [DOI] [PubMed] [Google Scholar]
- Crago S. S., Kulhavy R., Prince S. J., Mestecky J. Secretory component of epithelial cells is a surface receptor for polymeric immunoglobulins. J Exp Med. 1978 Jun 1;147(6):1832–1837. doi: 10.1084/jem.147.6.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M. M., Nagy B., Bazin H., Underdown B. J. Biliary transport of IgA: role of secretory component. Proc Natl Acad Sci U S A. 1979 Apr;76(4):2008–2012. doi: 10.1073/pnas.76.4.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goding J. W. Conjugation of antibodies with fluorochromes: modifications to the standard methods. J Immunol Methods. 1976;13(3-4):215–226. doi: 10.1016/0022-1759(76)90068-5. [DOI] [PubMed] [Google Scholar]
- Jackson G. D., Lemaître-Coelho I., Vaerman J. P., Bazin H., Beckers A. Rapid disappearance from serum of intravenously injected rat myeloma IgA and its secretion into bile. Eur J Immunol. 1978 Feb;8(2):123–126. doi: 10.1002/eji.1830080210. [DOI] [PubMed] [Google Scholar]
- Johns P., Stanworth D. R. A simple numerical method for the construction of isokinetic sucrose density gradients, and their application to the characterisation of immunoglobulin complexes. J Immunol Methods. 1976 Mar;10(2-3):231–252. doi: 10.1016/0022-1759(76)90174-5. [DOI] [PubMed] [Google Scholar]
- Kaartinen M. Liver damage in mice and rats causes tenfold increase of blood immunoglobulin A. Scand J Immunol. 1978;7(6):519–522. doi: 10.1111/j.1365-3083.1978.tb00487.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi K. Studies on human secretory IgA comparative studies of the IgA-bound secretory piece and the free secretory piece protein. Immunochemistry. 1971 Sep;8(9):785–800. doi: 10.1016/0019-2791(71)90446-0. [DOI] [PubMed] [Google Scholar]
- Lemaitre-Coelho I., Jackson G. D., Vaerman J. P. Relevance of biliary IgA antibodies in rat intestinal immunity. Scand J Immunol. 1978;8(5):459–463. doi: 10.1111/j.1365-3083.1978.tb00542.x. [DOI] [PubMed] [Google Scholar]
- Lemaître-Coelho I., Jackson G. D., Vaerman J. P. High levels of secretory IgA and free secretory component in the serum of rats with bile duct obstruction. J Exp Med. 1978 Mar 1;147(3):934–939. doi: 10.1084/jem.147.3.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaître-Coelho I., Jackson G. D., Vaerman J. P. Rat bile as a convenient source of secretory IgA and free secretory component. Eur J Immunol. 1977 Aug;7(8):588–590. doi: 10.1002/eji.1830070818. [DOI] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- McConahey P. J., Dixon F. J. A method of trace iodination of proteins for immunologic studies. Int Arch Allergy Appl Immunol. 1966;29(2):185–189. doi: 10.1159/000229699. [DOI] [PubMed] [Google Scholar]
- Mehta S. K., Plaut A. G., Calvanico N. J., Tomasi T. B., Jr Human immunoglobulin A: production of an Fc fragment by an enteric microbial proteolytic enzyme. J Immunol. 1973 Oct;111(4):1274–1276. [PubMed] [Google Scholar]
- Mullock B. M., Hinton R. H., Dobrota M., Peppard J., Orlans E. Endocytic vesicles in liver carry polymeric IgA from serum to bile. Biochim Biophys Acta. 1979 Oct 18;587(3):381–391. doi: 10.1016/0304-4165(79)90442-2. [DOI] [PubMed] [Google Scholar]
- Nagura H., Nakane P. K., Brown W. R. Translocation of dimeric IgA through neoplastic colon cells in vitro. J Immunol. 1979 Nov;123(5):2359–2368. [PubMed] [Google Scholar]
- Nakamura I., Ray A., Mäkelä O. Oligomeric IgA: the major component of the in vitro primary response of mouse spleen fragments. J Exp Med. 1973 Oct 1;138(4):973–988. doi: 10.1084/jem.138.4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlans E., Peppard J., Fry J. F., Hinton R. H., Mullock B. M. Secretory component as the receptor for polymeric IgA on rat hepatocytes. J Exp Med. 1979 Dec 1;150(6):1577–1581. doi: 10.1084/jem.150.6.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlans E., Peppard J., Reynolds J., Hall J. Rapid active transport of immunoglobulin A from blood to bile. J Exp Med. 1978 Feb 1;147(2):588–592. doi: 10.1084/jem.147.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORTER R. R. The hydrolysis of rabbit y-globulin and antibodies with crystalline papain. Biochem J. 1959 Sep;73:119–126. doi: 10.1042/bj0730119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo A. G., Lamm M. E., Plaut A. G., Frangione B. Secretory component is convalently bound to a single sub-unit in human secretory IgA. Mol Immunol. 1979 Jul;16(7):477–482. doi: 10.1016/0161-5890(79)90073-7. [DOI] [PubMed] [Google Scholar]
- Renston R. H., Jones A. L., Christiansen W. D., Hradek G. T., Underdown B. J. Evidence for a vesicular transport mechanism in hepatocytes for biliary secretion of immunoglobulin A. Science. 1980 Jun 13;208(4449):1276–1278. doi: 10.1126/science.7375938. [DOI] [PubMed] [Google Scholar]
- Socken D. J., Jeejeebhoy K. N., Bazin H., Underdown B. J. Identification of secretory component as an IgA receptor on rat hepatocytes. J Exp Med. 1979 Dec 1;150(6):1538–1548. doi: 10.1084/jem.150.6.1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socken D. J., Underdown B. J. Comparison of human, bovine and rabbit secretory component-immunoglobulin interactions. Immunochemistry. 1978 Jul;15(7):499–506. doi: 10.1016/0161-5890(78)90080-9. [DOI] [PubMed] [Google Scholar]
- Vaerman J. P., André C., Bazin H., Heremans J. F. Mesenteric lymph as a major source of serum IgA in guinea pigs and rats. Eur J Immunol. 1973 Sep;3(9):580–584. doi: 10.1002/eji.1830030911. [DOI] [PubMed] [Google Scholar]
- Vaerman J. P., Heremans J. F., Bazin H., Beckers A. Identification and some properties of rat secretory component. J Immunol. 1975 Jan;114(1 Pt 2):265–269. [PubMed] [Google Scholar]
- Zevenbergen J. L., May C., Wanson J. C., Vaerman J. P. Synthesis of secretory component by rat hepatocytes in culture. Scand J Immunol. 1980;11(1):93–97. doi: 10.1111/j.1365-3083.1980.tb00213.x. [DOI] [PubMed] [Google Scholar]