Abstract
The relative merits of fluorescein isothiocyanate (FITC) and lissamine rhodamine (RB 200) as labels for antibody in fluorescence microscopy were studied and compared by microphotometry, testing each fluorochrome under its own optimal conditions as far as possible, and at a similar range of dye:protein ratios. The antibody was sheep anti-human globulin, and the tissues stained with it were rat liver sections bearing human anti-nuclear factor on the nuclei. The findings were as follows:
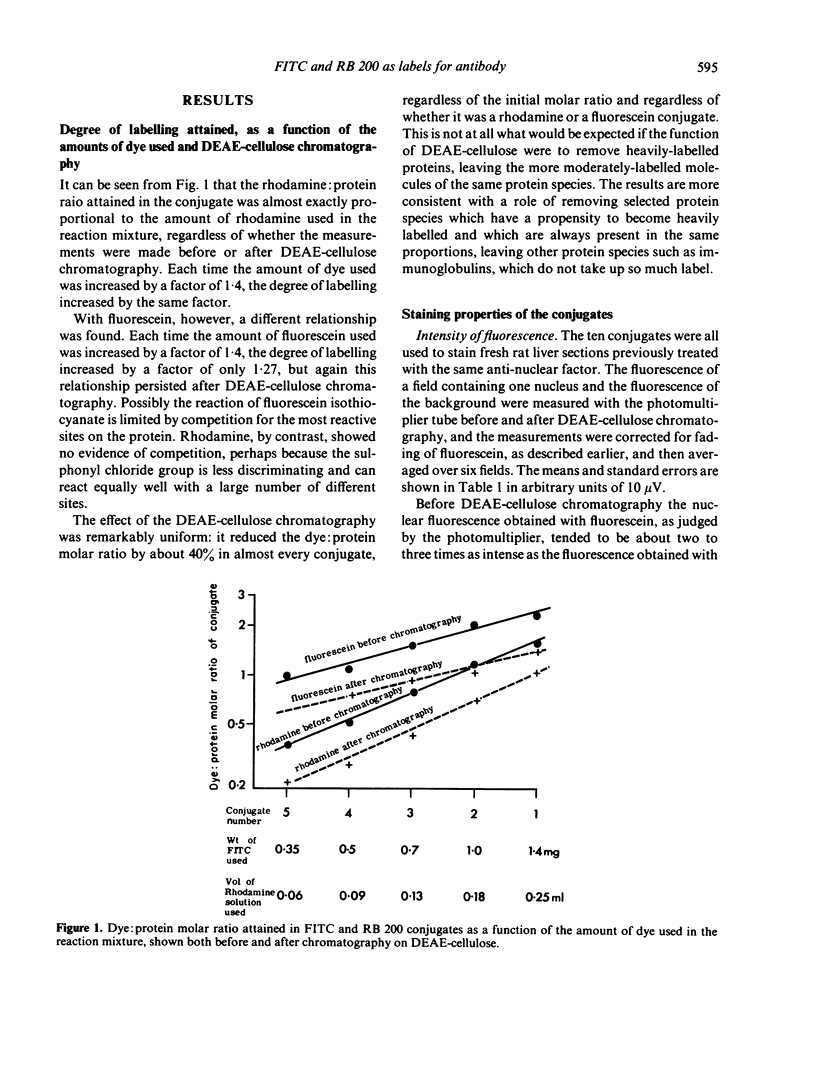
(i) the amount of RB 200 conjugating with protein was strictly proportional to the amount of the sulphonyl chloride derivative added to the reaction mixture; with increasing amounts of FITC in the reaction mixture, however, there was a less than proportional increase in the degree of conjugation.
(ii) Diethylaminoethyl (DEAE)-cellulose chromatography decreased the dye:protein ratio of the conjugates by 40% uniformly for both RB 200 and FITC, regardless of the initial dye:protein ratio.
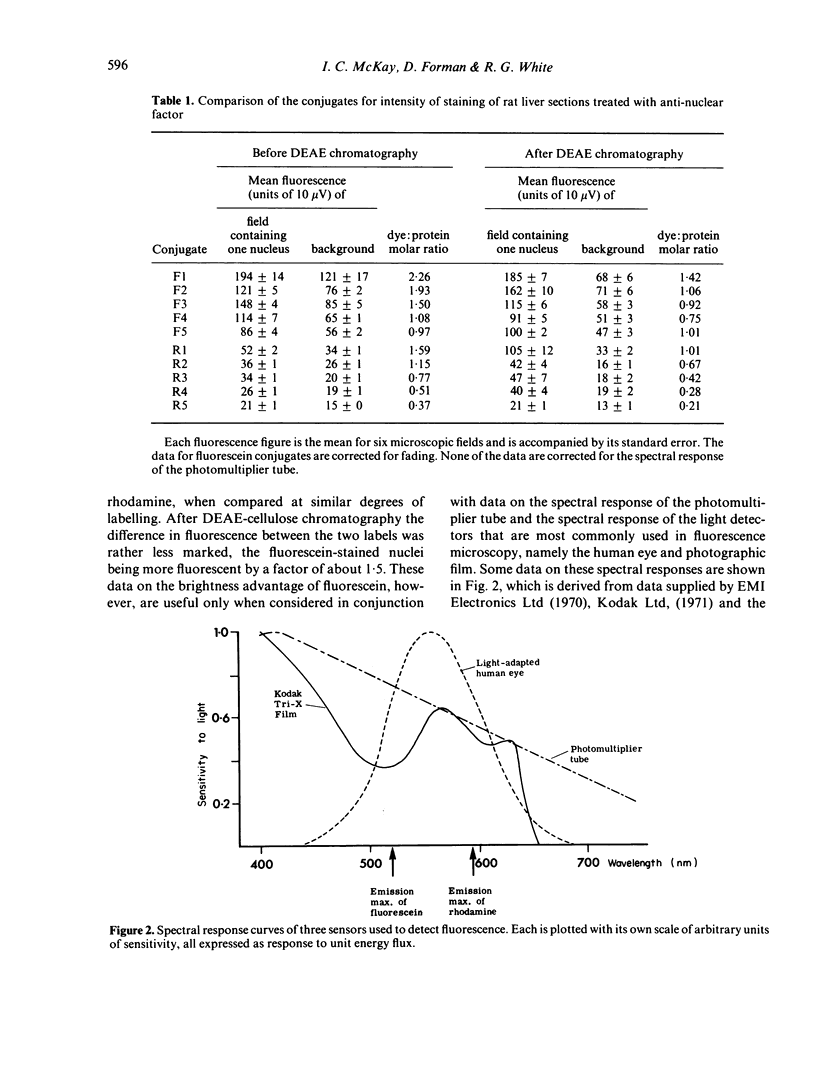
(iii) When corrections were made for spectral responses of photo-detectors, effects of optimizing the mountants, and benefits to rhodamine of changing from a Xenon to a mercury lamp, it was concluded that RB 200 conjugates could give brighter staining than FITC conjugates at similar dye:protein ratios.
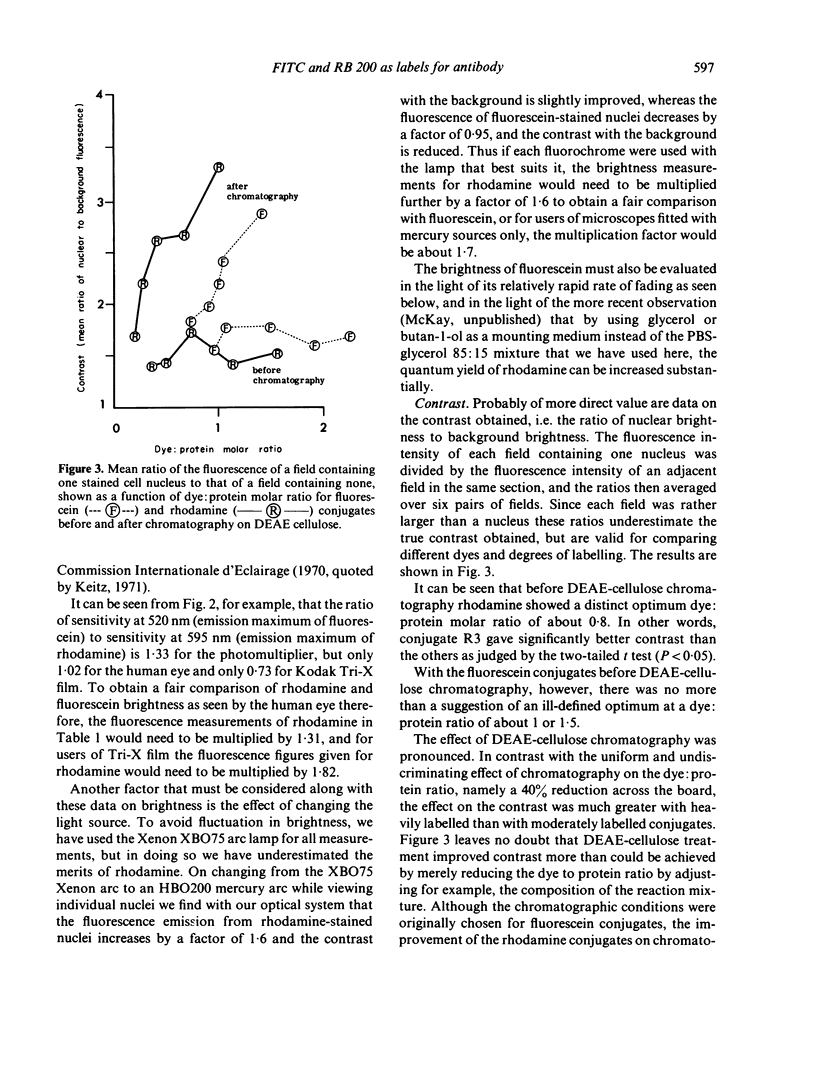
(iv) DEAE-cellulose chromatography greatly improved the contrast of the staining, especially with RB 200 conjugates.
(v) After chromatography, RB 200 consistently gave better contrast than FITC.
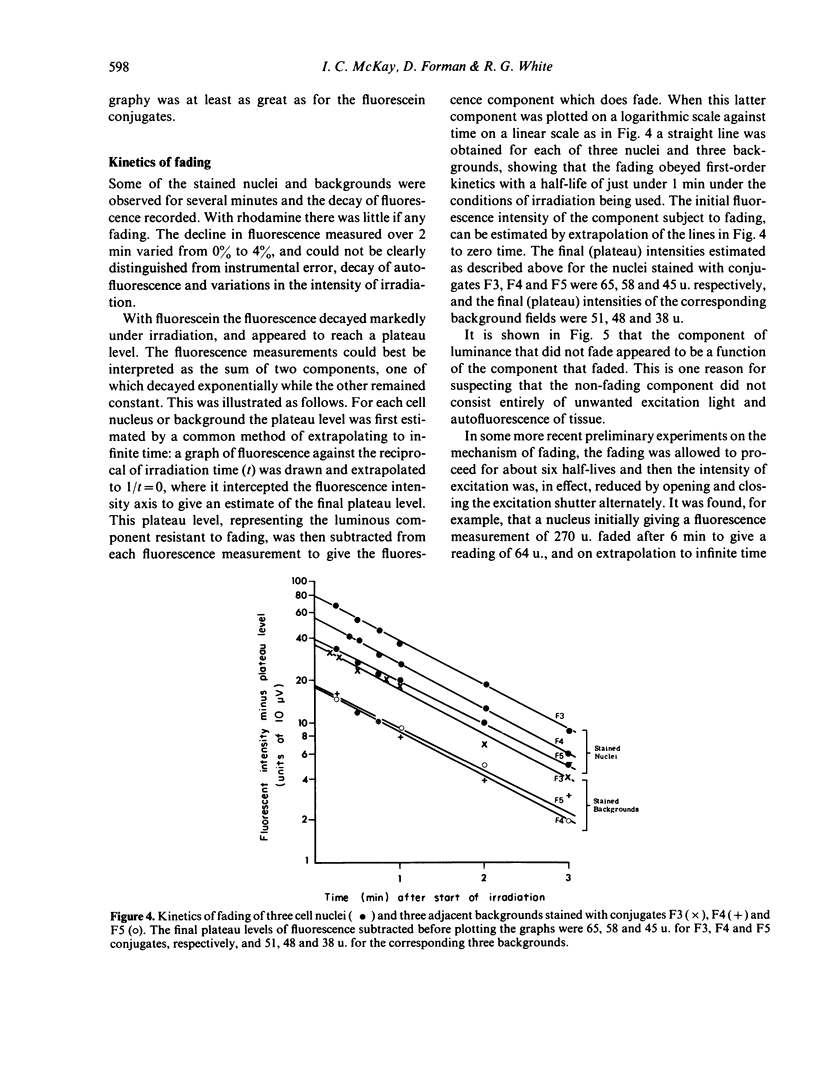
(vi) The fluorescence of rhodamine-stained sections did not fade demonstrably when irradiated for several minutes with green light.
(vii) The fluorescence of FITC-stained sections faded rapidly when irradiated with ultra-violet (u.v.)+blue light. The fluorescence appeared to contain two components, one fading with first-order kinetics with a half-life of about a minute under the experimental conditions used and the other not fading at all.
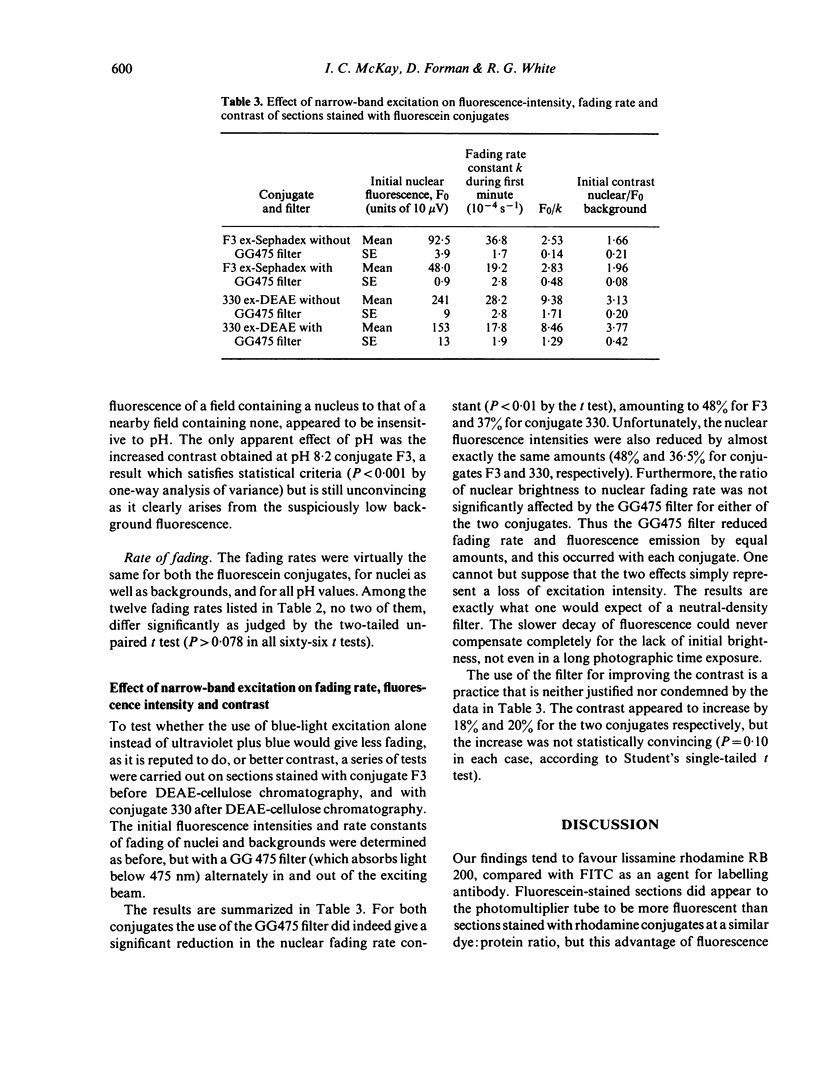
(viii) Raising the pH improved the fluorescence of FITC-stained sections but did not affect rates of fading.
(ix) Narrow-band excitation of FITC-stained sections with blue light instead of u.v.+blue reduced the rate of fading and the fluorescence intensity by equal amounts, an effect presumably due merely to loss of excitation intensity.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brighton W. D., Grulich R. An alternative lamp for fluorescence microscopy. Immunology. 1972 Feb;22(2):301–303. [PMC free article] [PubMed] [Google Scholar]
- CHADWICK C. S., McENTEGART M. G., NAIRN R. C. Fluorescent protein tracers; a trial of new fluorochromes and the development of an alternative to fluorescein. Immunology. 1958 Oct;1(4):315–327. [PMC free article] [PubMed] [Google Scholar]
- GOLDMAN M. Antigenic analysis of Entamoeba histolytica by means of fluorescent antibody. I. Instrumentation for microfluorimetry of stained amebae. Exp Parasitol. 1960 Feb;9:25–36. doi: 10.1016/0014-4894(60)90006-0. [DOI] [PubMed] [Google Scholar]
- HIRAMOTO R., BERNECKY J., JURAND J., HAMLIN M. THE EFFECT OF HYDROGEN ION CONCENTRATION ON FLUORESCENT LABELLED ANTIBODIES. J Histochem Cytochem. 1964 Apr;12:271–274. doi: 10.1177/12.4.271. [DOI] [PubMed] [Google Scholar]
- Hijmans W., Schuit H. R., Teiko Y., Schechter I. An immunofluorescence study on the specificity of antibodies synthesized in separate cells after the administration of an immunogen with double specificity. Eur J Immunol. 1972 Feb;2(1):1–4. doi: 10.1002/eji.1830020102. [DOI] [PubMed] [Google Scholar]
- Jobbágy A., Király K. Chemical characterization of fluorescein isothiocyanate-protein conjugates. Biochim Biophys Acta. 1966 Jul 27;124(1):166–175. doi: 10.1016/0304-4165(66)90325-4. [DOI] [PubMed] [Google Scholar]
- Kaufman G. I., Nester J. F., Wasserman D. E. An experimental study of lasers as excitation sources for automated fluorescent antibody instrumentation. J Histochem Cytochem. 1971 Aug;19(8):469–476. doi: 10.1177/19.8.469. [DOI] [PubMed] [Google Scholar]
- LEWIS V. J., BROOKS J. B. COMPARISON OF FLUOROCHROMES FOR THE PREPARATION OF FLUORESCENT-ANTIBODY REAGENTS. J Bacteriol. 1964 Nov;88:1520–1521. doi: 10.1128/jb.88.5.1520-1521.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nairn R. C., Herzog F., Ward H. A., De Boer W. G. Microphotometry in immunofluorescence. Clin Exp Immunol. 1969 Jun;4(6):697–705. [PMC free article] [PubMed] [Google Scholar]
- Ploem J. S. A study of filters and light sources in immunofluorescence microscopy. Ann N Y Acad Sci. 1971 Jun 21;177:414–429. doi: 10.1111/j.1749-6632.1971.tb35070.x. [DOI] [PubMed] [Google Scholar]
- Wells A. F., Miller C. E., Nadel M. K. Rapid fluorescein and protein assay method for fluorescent-antibody conjugates. Appl Microbiol. 1966 Mar;14(2):271–275. doi: 10.1128/am.14.2.271-275.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]


