Abstract
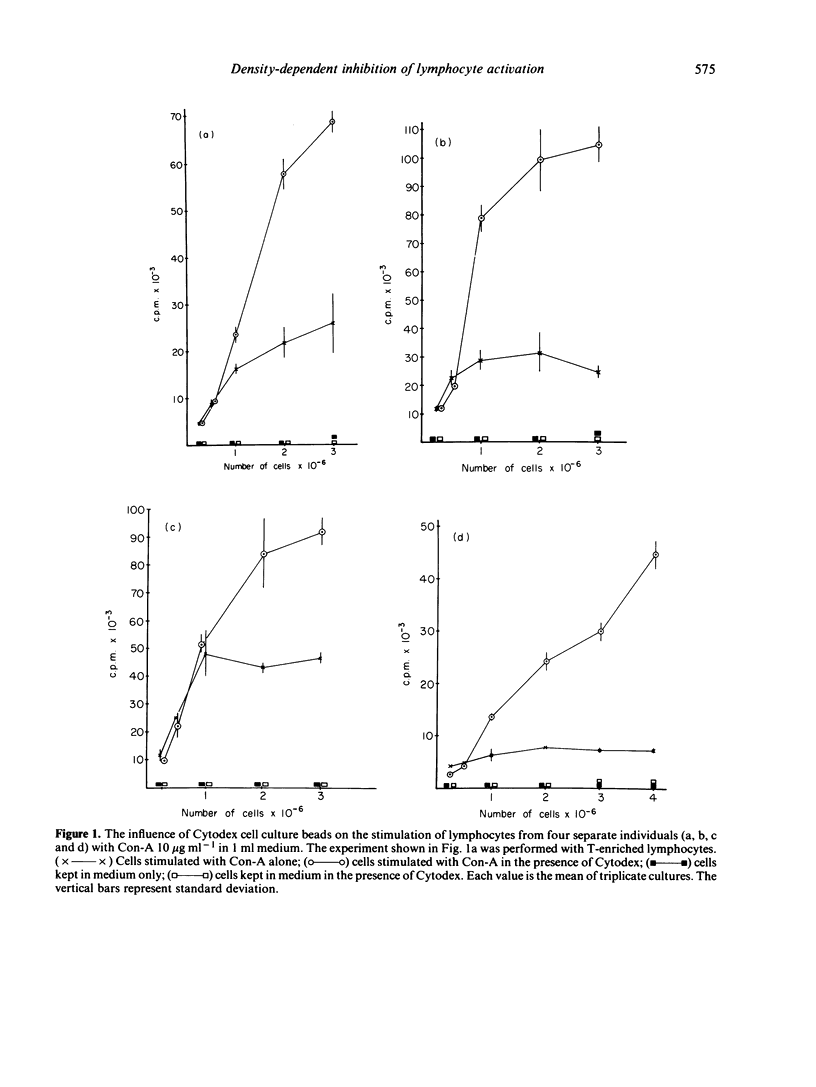
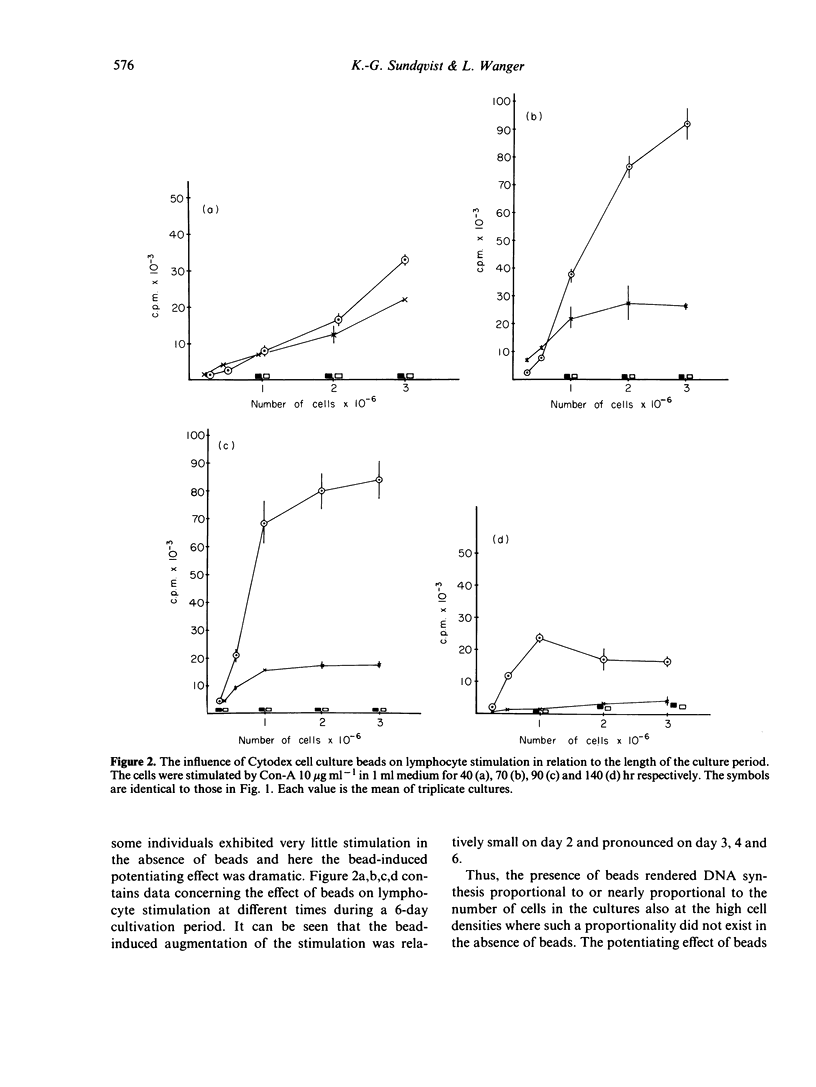
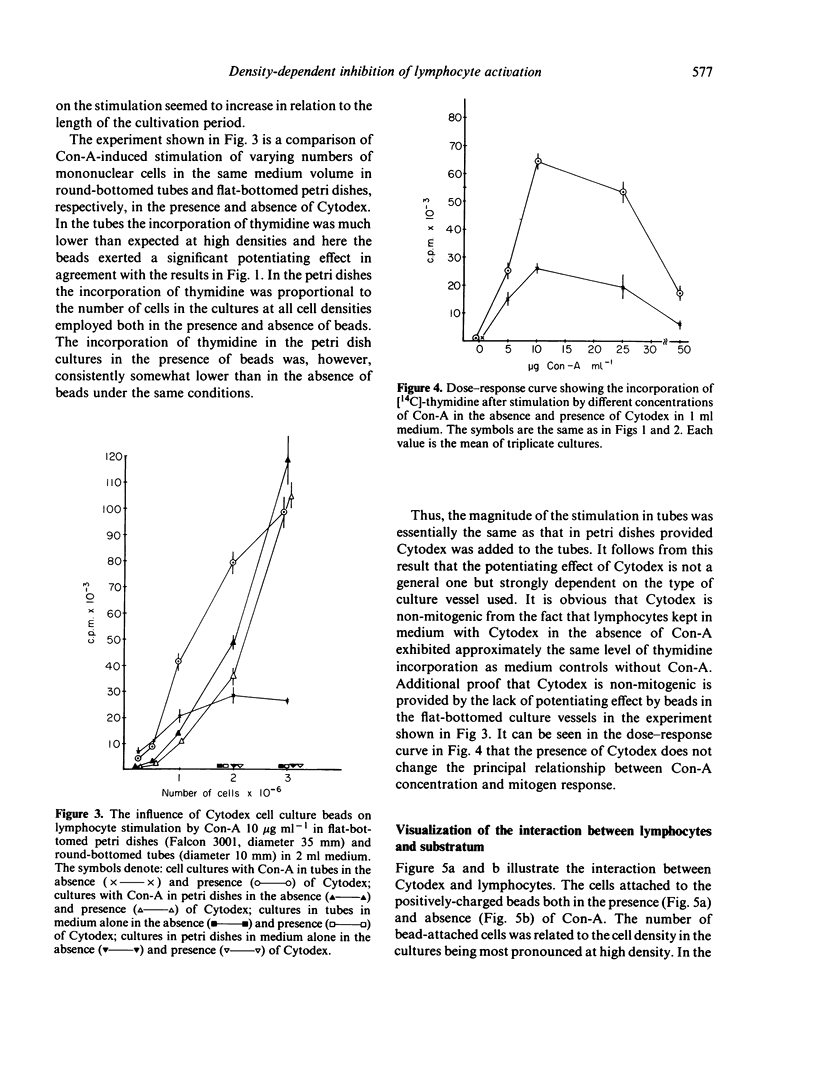
Varying numbers of human blood lymphocytes were stimulated by concanavalin A (Con-A) in a constant medium volume. At 'low' cell densities DNA synthesis was proportional to the number of cells in the cultures whereas at 'high' densities DNA synthesis was considerably lower than expected. In the presence of non-mitogenic microcarrier beads, (diameter 195 micrometer) to which the cells attached, DNA synthesis was proportional or nearly proportional to the cell number in the cultures also at 'high' cell densities. The potentiating effect of beads on lymphocyte stimulation was particularly noteworthy in individuals showing a weak mitogen response. Another approach that yielded proportionality between DNA synthesis and cell number both at 'low' and 'high' cell densities was the use of culture vessels with a larger bottom area. Under such conditions the presence of beads did not augment DNA synthesis. These results suggest that the availability of non-cellular adhesive surface is a major limiting factor and cell density a major regulating factor in the control of lymphocyte activation. Anchorage of the cells to a surface may modulate the density dependent 'growth control mechanism' indirectly via an influence on cell-cell interaction. An alternative less-likely interpretation is that the contact with non-cellular surfaces directly gives rise to regulatory responses in lymphocytes or accessory cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Ze'ev A., Farmer S. R., Penman S. Protein synthesis requires cell-surface contact while nuclear events respond to cell shape in anchorage-dependent fibroblasts. Cell. 1980 Sep;21(2):365–372. doi: 10.1016/0092-8674(80)90473-0. [DOI] [PubMed] [Google Scholar]
- Farrant J., Knight S. C. Help and suppression by lymphoid cells as a function of cellular concentration. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3507–3510. doi: 10.1073/pnas.76.7.3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J., Moscona A. Role of cell shape in growth control. Nature. 1978 Jun 1;273(5661):345–349. doi: 10.1038/273345a0. [DOI] [PubMed] [Google Scholar]
- Gillis S., Smith K. A. Long term culture of tumour-specific cytotoxic T cells. Nature. 1977 Jul 14;268(5616):154–156. doi: 10.1038/268154a0. [DOI] [PubMed] [Google Scholar]
- Habu S., Raff M. C. Accessory cell dependence of lectin-induced proliferation of mouse T lymphocytes. Eur J Immunol. 1977 Jul;7(7):451–457. doi: 10.1002/eji.1830070710. [DOI] [PubMed] [Google Scholar]
- Hersh E. M., Harris J. E. Macrophage-lymphocyte interaction in the antigen-induced blastogenic response of human peripheral blood leukocytes. J Immunol. 1968 Jun;100(6):1184–1194. [PubMed] [Google Scholar]
- Larsson E. L., Coutinho A. The role of mitogenic lectins in T-cell triggering. Nature. 1979 Jul 19;280(5719):239–241. doi: 10.1038/280239a0. [DOI] [PubMed] [Google Scholar]
- Moorhead J. F., Connolly J. J., McFarland W. Factors affecting the reactivity of human lymphocytes in vitro. I. Cell number, duration of culture and surface area. J Immunol. 1967 Aug;99(2):413–419. [PubMed] [Google Scholar]
- Peters J. H. Contact cooperation in stimulated lymphocytes. I. Influence of cell contact on unspecifically stimulated lymphocytes. Exp Cell Res. 1972 Sep;74(1):179–186. doi: 10.1016/0014-4827(72)90495-8. [DOI] [PubMed] [Google Scholar]
- Schmidtke J. R., Hatfield S. Activation of purified human thymus-derived (T) cells by mitogens. II. Monocyte- macrophage potentiation of mitogen-induced DNA synthesis. J Immunol. 1976 Feb;116(2):357–362. [PubMed] [Google Scholar]
- Stoker M. G., Rubin H. Density dependent inhibition of cell growth in culture. Nature. 1967 Jul 8;215(5097):171–172. doi: 10.1038/215171a0. [DOI] [PubMed] [Google Scholar]
- Stoker M., O'Neill C., Berryman S., Waxman V. Anchorage and growth regulation in normal and virus-transformed cells. Int J Cancer. 1968 Sep 15;3(5):683–693. doi: 10.1002/ijc.2910030517. [DOI] [PubMed] [Google Scholar]
- Sundqvist K. G., Wanger L. Anchorage and lymphocyte function. Contact-induced augmentation of T-cell activation. Immunology. 1980 Dec;41(4):883–890. [PMC free article] [PubMed] [Google Scholar]
- Wanger L., Sundqvist K. G. Contact-induced modification of lymphocyte morphology. Biochem Soc Symp. 1980;45:65–73. [PubMed] [Google Scholar]
- Zetterberg A., Auer G. Proliferative activity and cytochemical properties of nuclear chromatin related to local cell density of epithelial cells. Exp Cell Res. 1970 Sep;62(1):262–270. doi: 10.1016/0014-4827(79)90527-5. [DOI] [PubMed] [Google Scholar]