Abstract
Objective:
To compare surface cooling and deep cooling produced by 3 common forms of cryotherapy.
Design and Setting:
We used a 3 × 4 × 4 factorial with repeated measures on measurement depth and treatment. Independent variables were measurement depth (surface, fat + 1 cm, and fat + 2 cm), treatment (ice bag, Wet-Ice, Flex-i-Cold, and control), and treatment order (first, second, third, and fourth). The lowest temperature recorded was the dependent variable. The treatment order was counterbalanced using a Latin square. Data were analyzed with a repeated-measures analysis of variance.
Subjects:
Fifteen collegiate volunteers who were free of lower extremity abnormalities.
Measurements:
Thigh skin and thigh intramuscular temperatures (1- and 2-cm subadipose) were measured at 30-second intervals both before and during the 30-minute treatments using fine-wire implantable and surface thermocouples. The coldest recorded temperatures were analyzed.
Results:
Statistical differences were observed for the depth-by-treatment interaction as well as for the depth and treatment main effects. During cold treatments, superficial depths were colder than deeper depths, and all cold treatments were colder than controls at all depths. For the interaction effect at both the skin surface and at 1-cm subadipose, the ice-bag and Wet-Ice treatments were colder than the Flex-i-Cold treatment. For the interaction at 2-cm subadipose, the cold treatments did not differ from each other. Order of treatments did not produce a significant effect.
Conclusions:
During a 30-minute cryotherapy treatment, modalities that undergo a phase change caused lower skin and 1-cm intramuscular temperatures than cold modalities that do not possess these properties. These differences were not seen at 2-cm subadipose but may become apparent with longer treatments.
Keywords: Wet-Ice, Flex-i-Cold, cryotherapy, thermocouple
Cryotherapy, the therapeutic use of cold, is the most commonly used modality in the acute management of musculoskeletal injuries.1–3 The primary rationale for this use of cold involves the demonstrated ability of cold to reduce the metabolic rate of a tissue.2–6 This metabolic reduction may allow an otherwise uninjured tissue to survive a postinjury period of ischemia or be protected from damaging enzymatic reactions that may accompany injury.2–4
At this time, there is no clear consensus on the optimum application technique for acute cryotherapy. It is generally assumed that immediate cryotherapy application will be more beneficial than delayed application based on the assumption that the sooner the metabolic rate is reduced after injury, the less the secondary damage. Thus, cryotherapy techniques that provide more rapid cooling of tissues may offer some advantage over slower cooling techniques. Similarly, although there is only limited support in the literature,7 it is generally assumed that greater cooling leads to more profound metabolic suppression, suggesting that cryotherapy modalities that produce lower temperatures are more efficacious.
A large body of literature1,3,8–20 describes both surface and intramuscular (IM) temperatures during various forms of cryotherapy. From a cursory examination of this literature, it would appear that the cooling efficacy of cryotherapy depends on many factors, including the duration of cold application,12,17 the anatomical location of the cryotherapy treatments,1,3,8,9,11–15,19 the use of compression,1,16,20 the level of prior or subsequent physical activity,3,15,19 and the specific mode of cryotherapy used.1,11,14,18 Although comparisons of different modes of cryotherapy can be made across different studies, very few studies18 involved direct comparisons of different modes of cryotherapy.
Various modes of cryotherapy are commonly used. These modalities have different thermodynamic properties, which may result in different cooling efficacies. For example, ice undergoes a physical change of state (solid to liquid) during use, whereas frozen gel packs do not. Although basic thermodynamics would lead us to believe that colder IM temperatures are produced by modalities changing state (due to greater heat absorption) than by modalities that do not change state,3 we found no published studies in which this question had been examined. Similarly, with some cold modalities, heat transfer occurs primarily through conduction, whereas with others, conduction and evaporation both dissipate heat. To date, the cooling efficacies of different modes of cryotherapy with these varied thermodynamic properties have not been well described. Therefore, the purpose of this study was to compare the cooling efficacies of several commonly used forms of cryotherapy: bags of crushed ice, commercially available ice packs (Wet-Ice, Peak Performance, Waverly, IA), and commercially available frozen gel packs (Flex-i-Cold, Cramer Products Inc, Gardner, KS).
METHODS
Experimental Design
The design for this study was a fixed-model 3 × 4 × 4 factorial with repeated measures on the first (depth) and second (mode) independent variables. The independent variables were depth of temperature measurement (3 levels: surface, 1-cm subadipose, 2-cm subadipose), treatment mode (4 levels: ice bag, Wet-Ice, Flex-i-Cold, control), and ordinal position of treatment (4 levels: first, second, third, fourth). Treatment order was counterbalanced using a Latin square. The dependent variable was the lowest temperature recorded.
Subjects
The subjects for this study were 15 volunteers (age = 21.7 ± 1.2 years, height = 177.8 ± 8.9 cm, mass = 75.2 ± 3.4 kg, anterior thigh skinfold = 19.3 ± 4.1 mm), men and women, from a university student population. Each subject completed a health status questionnaire and gave written informed consent before participating in the study. The procedures in this study were approved by the university's human subjects committee.
Instrumentation
Temperature data were collected using type-T (copper/constantan) thermocouples interfaced with a computer-based thermocouple thermometer (Iso-Thermex-16, Columbus Instruments, Columbus, OH). Measurements made with this equipment are accurate to within 1%.21 Surface and ambient temperatures were measured using 30-gauge, exposed-junction thermocouples with Kapton-insulated leads (TX-31, Columbus Instruments). Intratissue temperatures were measured using implantable, ungrounded-junction thermocouples (diameter = 0.4 mm) with Teflon-PTFE–insulated leads (TX-23-21, Columbus Instruments) implanted using sterile, 21-gauge hypodermic needles. Implantable thermocouples were disinfected by immersion in a CidexPlus 3.4% glutaraldehyde solution22 (Johnson & Johnson Medical, Arlington, TX) for 20 minutes.
Three modes of cryotherapy were used in this study. They included bags of crushed ice (1 kg ice in a 4-L plastic bag, air evacuated before closing) applied with a 15-cm double-length elastic wrap, Wet-Ice applied with its attached elastic bands, and Flex-i-Cold applied with elastic wraps in the same manner as the ice bags. Wet-Ice was chosen because the melting ice from the pack comes in contact with the skin through a terrycloth panel, allowing for evaporative cooling and heat transfer through a wet interface. Flex-i-Cold was chosen because it is a frozen gel pack and does not change state physically.
Typical pressures for elastic wraps applied to the thigh are approximately 45 to 50 mm Hg.1,20 Therefore, compression during all 3 modes of cryotherapy was standardized at 45 mm Hg using a manometer with a bladder. Controls received neither cryotherapy nor compression.
Procedures
Subjects assumed a supine position on a standard treatment table. In an effort to minimize the likelihood of infection, the hair on a 6- by 6-cm area of the anterior right thigh was clipped using barber-style hair clippers (Wahl Inc, Sterling, IL) and cleaned with a povidone-iodine surgical scrub solution for 30 seconds. The center point of this area was located at the midpoint of a line between the superior border of the patella and the anterior superior iliac spine. The cold modalities actually covered a considerably larger area than that clipped on the thigh. Four thermocouples (2 surface and 2 implantable) were used for each subject and arranged in a 2- by 2-cm grid at the center of the cleaned and clipped area of the thigh.
Thermocouple insertion depths relative to adipose thickness were calculated using a method previously described1: the depths were ([skinfold/2] + 1 cm) or ([skinfold/2] + 2 cm), assuming skinfold/2 represents the thickness of the superficial adipose layer. The insertion depths were controlled by means of pen marks made on the thermocouple leads, 6 cm from the tips. The distance from the skin surface to these marks was measured and subtracted from 6 cm, with the difference representing the actual inserted depth of the thermocouple. Thermocouples were inserted slightly deeper than needed and then adjusted to the correct depth.
The surface thermocouples were secured to the skin using Dermaclear tape (Johnson & Johnson). The 2 tissue-implantable thermocouples were implanted using 3.8-cm (1.5 in) × 21-gauge sterile hypodermic needles inserted perpendicular to the skin. An investigator wearing examination gloves and using universal precautions inserted the hypodermic needles to a calculated depth (1- or 2-cm subadipose); then the needles were withdrawn along the thermocouple leads, leaving the flexible thermocouples in place.
The thermocouple leads were then taped to the thigh of the subject with Dermaclear tape at the insertion site and approximately 6 cm from the insertion site to prevent the leads from being disturbed. By using uniform amounts of tape covering an equal area for each individual treatment, we controlled the potential insulating effect of the tape. By controlling the amount and area of the tape, it was assumed that a uniform effect would be produced across all treatments and, therefore, would not interfere with our ability to discriminate among treatments.
The thermocouples have miniconnectors on their Iso-Thermex interface ends, which prevent the insertion needles from being removed from the thermocouple leads until the entire lead is withdrawn from the subject at the conclusion of the session. Because the needles remained on the thermocouple leads during measurement, they posed a risk for an accidental needle-stick injury. Therefore, after the needles were withdrawn from the subjects and were slid up the lead wires and out of the way, they were taped to the Iso-Thermex to minimize the likelihood of accidental needle sticks.
Treatment Protocol
Once the thermocouples were in place, instantaneous temperature measurements were taken at 30-second intervals for the duration of the experimental period (32 minutes). Subjects rested in a supine position for 3 minutes before the experimental treatment while baseline temperature data were recorded.
At the end of the 2-minute baseline period, 1 of the 4 experimental treatments described above was applied for 30 minutes. Subsequent treatment sessions were spaced a minimum of 48 hours apart. As noted earlier, all 3 cryotherapy treatments were applied with similar compression pressures, as measured by a manometer. The bladder of the manometer was placed at the center of the distal margin of the clipped area, approximately 2 cm from the closest thermocouple, so that it would not act as a thermal insulator for any thermocouple.
Thermocouple Removal and Posttest Instructions
After 30 minutes of treatment, the thermocouples were individually removed, cleaned, and disinfected with CidexPlus immersion.22 A topical antiseptic solution (bacitracin) was applied to the insertion sites, which were then bandaged with sterile adhesive bandages. Needles were disposed of in appropriate containers. Subjects were given instructions for care of the insertion wounds and were advised to contact the investigator or the Student Health Center if they experienced any problems with the wounds. Subjects reported no adverse events or infections.
Statistical Analysis
We performed an initial, repeated-measures analysis of variance (ANOVA) to compare baseline temperatures across treatments. The lowest temperature data were compared across treatments, treatment depths, and treatment orders using a repeated-measures ANOVA with simple main-effects testing and the Sidak t pairwise post hoc comparisons when appropriate. The error rate was set a priori at P = .05.
RESULTS
Baseline and lowest temperature data are found in the Table. Baseline temperatures did not differ across treatments (F6,3 < 1, P = .512).
Baseline and Lowest Skin and Intramuscular Temperatures (°C) at the Thigh for Several Cold Modalities
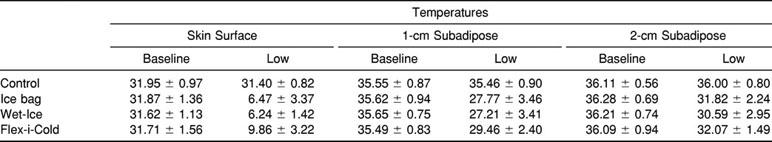
For the cold conditions at both IM depths, the lowest temperatures occurred within 2 samples (60 seconds) of the final sample, and none differed from the final temperature by more than 0.15°C (Figures 1 through 3). At the skin surface, the Flex-i-Cold treatment produced its coldest temperature at roughly one third of the way through the treatment (at 11.0 ± 2.7 minutes), whereas the ice and Wet-Ice treatments were coldest within 60 seconds of the end of the treatment. Interestingly, at all measurement depths, the control temperatures were coldest within the first minute of the measurement.
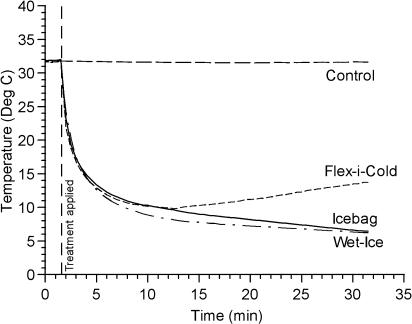
Figure 1.
Temperature change (°C) across time at the skin surface.
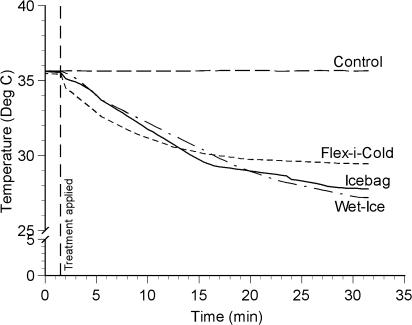
Figure 3.
Temperature change (°C) across time at 2-cm subadipose.
A significant interaction effect was observed for depth by treatment (F6,3 = 56.38, P = .04, η2 = 0.991, 1 − β = 1.0). Significant main effects were observed for both depth (F2,7 = 377.75, P < .005, η2 = 0.991, 1 − β = 1.0) and treatment (F3,6 = 375.16, P < .005, η2 = 0.995, 1 − β = 1.0). Because the interaction effect was observed for the same independent variables as the main effects, the interaction is more meaningful; therefore, primary attention should be given to the interaction rather than to the main effects. No effect for order of treatments was observed (F3,6 < 1, P = .86, η2 = 0.11, 1 − β = 0.23).
For the depth-by-treatment interaction effect at the skin surface (see Figure 1), all 3 cold treatments produced cooler temperatures than did the control. Also, the 2 ice-based treatments (ice bag and Wet-Ice) produced cooler temperatures than did the gel pack (Flex-i-Cold). The results of the ice-bag and Wet-Ice treatments were not significantly different.
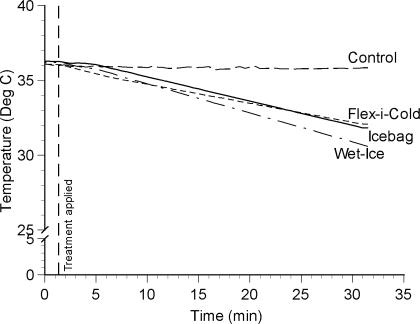
For the depth-by-treatment interaction at 1-cm subadipose (see Figure 2), all 3 cold treatments produced cooler temperatures than did the control. Also, the ice-based treatments produced cooler temperatures than did the frozen gel pack. The ice-bag and Wet-Ice treatments did not differ from each other.
Figure 2.
Temperature change (°C) across time at 1-cm subadipose.
For the depth-by-treatment interaction at 2-cm subadipose (see Figure 3), all 3 cold treatments produced cooler temperatures than did the control. The 3 cold treatments did not differ from each other.
DISCUSSION
In cryotherapy research, there is perhaps no more important question than the optimum temperature and duration of cryotherapy needed to minimize the effects of acute trauma.2 Unfortunately, this question remains unanswered; in fact, it is largely unexamined at this point. Although we know that cryotherapy is effective in managing injury,2–4,7,8,16,23 we still do not know many of the important physiologic details that will allow us to maximize its beneficial effects.
Colder is Better?
In the absence of definitive research, we generally assume that better outcomes result from greater and faster cooling. Some minimal evidence suggests that colder is better, up to a point.7 In a limb-replantation study, Sapega et al7 examined the adenosine triphosphate (ATP)-sparing effect of cryotherapy in the musculature of amputated cat hind limbs in vitro. Between 22°C and 5°C, colder storage temperatures resulted in greater ATP sparing. At 1°C, however, ATP usage actually increased. Unfortunately, this study is of limited value in acute cryotherapy because IM temperatures during cryotherapy in vivo in humans rarely fall below 20°C.1
Because we are operating with the assumption that colder is better, the purpose of our study was to compare the magnitude of cooling produced by several commonly used forms of cryotherapy, namely bags of crushed ice, commercially available ice packs (Wet-Ice), and commercially available frozen gel packs (Flex-i-Cold). These specific cold modalities were chosen because they possess slightly different thermodynamic properties. Our intention was to determine whether the differences in thermodynamic properties would lead to differences in tissue-cooling abilities. From our results, it appears that the different thermodynamic properties of these forms of cryotherapy led to different surface or IM temperatures during 30-minute applications.
Cryotherapy Thermodynamics
To discuss the findings presented in this article, some brief background information on the thermodynamics of cryotherapy is warranted. First and most importantly, heat transfer is always unidirectional from high heat to low heat.24,25 Therefore, cryotherapy modalities do not transfer cold to the tissues because cold is not transferable. Instead, tissues warm the cold modalities by losing heat to them. Although this may seem like a matter of semantics, it actually has some important implications for the efficacy of cryotherapy. Because heat transfer is unidirectional, cold modalities work by absorbing heat from their immediate environment, particularly from the tissues being treated. Similarly, deep tissues are cooled by losing their heat to more superficial tissues, which have been cooled by the modalities. The transfer of heat and the capacity of the cold modality to absorb this heat are therefore paramount in determining the modality's effectiveness.
The transfer of heat from one body to another depends on several factors.3,25 Some of the most important of these are the relative masses of the bodies, the size of the contact area, the difference in starting temperatures, the heat capacity (Cp) of each material, and in the case of cryotherapy, rewarming of the tissue from its own metabolic activity and from perfusion.
The Cp of a material (also known as specific heat) is the quantity of heat required to raise a specific mass of the material by a specific number of degrees. Generally, it is expressed as the amount of heat to raise 1 kg of material by 1K (or by 1°C because the units are the same).24,25 The greater the Cp of the material, the more heat required to change its temperature. The Cp of ice (at 0°C) is 2090 J·kg−1·°C−1, whereas the Cp of water (at 0°C) is 4190 J·kg−1·°C−1.24 Thus, ice warms more easily than water.
A related property of materials is the enthalpy (heat) of fusion (ΔfusH), which is the quantity of heat required to make the material change phase from solid to liquid. Fusion (melting) occurs when a specific quantity of heat is added to a solid material whose temperature is at the melting point for that material (eg, ice at 0°C). Note also that fusion occurs without a change in temperature,25 that is, the solid immediately before melting and the liquid immediately after have the same temperature. In the case of ice water, the melting temperature is 0°C and the enthalpy of fusion (ΔfusH) is 333 000 J·kg−1.24,25 Note that the ΔfusH value is much greater than the Cp value. In fact, for water, it is nearly 80 times larger than the Cp value.
The concepts of heat capacity and enthalpy of fusion are important because they greatly affect the ability of a cold modality to absorb heat and should therefore be useful in predicting tissue cooling. For example, assume we begin with a 1-kg ice bag and a 1-kg gel pack (eg, Flex-i-Cold), both at 0°C. If we wanted to warm each by a single degree (to 1°C), we would add heat equal to 1 Cp for the gel pack but not for the ice bag. Because ice goes through fusion at 0°C, we would need to add heat equivalent to 1 ΔfusH to make it change from ice to water, then add heat equivalent to 1 Cp to raise it by 1°C. In other words, because the ice must go through fusion, we would need to add roughly 80 times more heat to raise the temperature by 1°C than if it did not go through fusion. Because ice goes through a change of physical state by melting, we should expect that ice-based cold modalities absorb much more heat than gel-based cold modalities (which do not melt) during cryotherapy treatments (even though gel packs often have colder initial temperatures). Greater ability of a modality to absorb heat should produce cooler temperatures in the treated tissues. Knight3 eloquently pointed this out in his classic text and used it as a rationale for recommending ice bags over gel packs. Unfortunately, although this argument was very strong from a thermodynamics standpoint, it had not been examined in the context of IM temperatures during cryotherapy until now.
One last thermodynamic principle that must be addressed relates to the mode of heat transfer. Cryotherapy modalities absorb heat through conduction, convection, evaporation, or some combination thereof. Both ice bags and gel packs absorb heat principally through conduction, that is, through direct contact with the treatment area. Cold baths absorb heat through conduction and may also absorb heat through convection in the event that the water is moving, as when we turn on the jets to a whirlpool. Some cold modalities absorb heat through conduction and allow the water produced by the melting ice to evaporate (to a limited degree). One such example is Wet-Ice, a commercially available fabric bag to which crushed ice is added. One of the panels of this bag is made from terrycloth, allowing the water from the melted ice to soak through. This makes for a wet interface, which should allow for greater thermal conduction than would be seen with a dry interface, because water has a relatively large thermal conductivity.24 To demonstrate the difference in conduction to yourself, try standing in a 16°C (60°F) room versus a 16°C (60°F) swimming pool and observe your reaction. From a thermodynamic standpoint, the addition of the better thermal conduction and the potential for additional evaporative cooling might potentially produce lower IM temperatures than those observed with typical ice bags. For this reason, we examined Wet-Ice.
Our Observations
For all measurement depths and treatments, we observed temperatures similar to those reported by others during cryotherapy to the thigh.1,12,26–28 We observed a depth-by-treatment interaction in this study. We interpreted this interaction through the use of simple main-effects testing, breaking down the effect by depth and looking for a difference between the treatments.
At the skin surface (see Figure 1), we observed that both the ice-based treatments (ice bag and Wet-Ice) produced lower temperatures than were observed with the gel pack (Flex-i-Cold). This fits well with the enthalpy of fusion principle because both of the ice-based treatments melted to varying degrees during the treatments, and this phase change resulted in the absorption of a great deal of heat. Interestingly, the skin temperature after the ice-bag treatment did not differ from the temperature with the Wet-Ice treatment. Apparently, the difference in thermal conduction and possible evaporation with the Wet-Ice was not great enough for a meaningful difference from typical ice bags at this depth.
At 1-cm subadipose, we observed a similar effect, with both ice-based treatments leading to cooler IM temperatures than those observed with the gel pack. Again, this fits well with our understanding of basic thermodynamics and enthalpy of fusion. As was the case at the skin surface, the 2 ice-based treatments did not differ statistically from each other. Again, it appears that the difference in thermal conduction and evaporative cooling was not meaningful in terms of reducing IM temperature.
At 2-cm subadipose, the effect was somewhat different from that observed at more superficial temperatures. At this depth, the 2 ice-based treatments did not differ from the cold gel pack or from each other. This does not fit well with the basic thermodynamic principles we have discussed and, at first glance, is somewhat puzzling. It would appear that the thermodynamics are different at 2-cm subadipose from those at more superficial depths.
For any tissue to become cool, heat loss must exceed heat gain. In the more superficial tissues, the difference between loss (to the cold modality) and gain (from metabolism and perfusion) during cryotherapy is reasonably large. However, as we look at tissues that are a greater distance from the cold modality, the relative difference between loss and gain becomes smaller as a result of the insulating effects of other tissues.
At the skin, the tissue loses heat directly to the modality through conduction. The relatively large temperature difference between the skin and the modality leads to a rather marked cooling of the skin. Looking deeper, the more superficial layers of muscle (1 cm) lose heat through conduction to the cooler tissues just superficial to them (eg, the skin). Although it is less than that at the skin surface, the temperature gradient between the superficial muscle and the skin is still large enough to lead to lower muscle temperature. In deeper muscle tissue (2 cm), heat is lost through conduction to the slightly cooler superficial muscle layers. This gradient is still large enough for the deeper tissues to be cooled, but the magnitude of temperature change is considerably smaller than that at more superficial depths.
Our observations fit well with this concept. At the skin surface, the overall temperature decline from baseline values was approximately 25°C, and the cryotherapy temperature difference between the ice-based modalities and the gel pack was approximately 2°C (see Table). These differences are not that high in deeper tissues. For example, at 1-cm subadipose, the overall decline in temperature was a more modest 6°C to 8°C, and the difference between ice-based modalities and the gel pack was approximately 1.7°C. When we examine temperatures at 2-cm subadipose, the overall decline in temperature was only about 4.5°C, and differences between the ice-based modalities and gel packs became insignificant. It appears that the insulating effects of the tissues dilute the net loss of heat to the cold modality and are substantial enough to reduce the differences seen between different modalities.
A related concept has to do with the thickness of the adipose tissue at the cryotherapy site. In a recent study, Otte et al29 observed that the thickness of the adipose tissue makes a substantial difference in the cooling time required during cryotherapy. As the subject's adipose thickness increased, the cooling time required to produce a standard temperature effect also increased quite dramatically (skinfold thickness of 20 mm or less required 20 minutes, whereas skinfolds of 20–30 mm required 38 minutes, and skinfolds of 30–40 mm required 59 minutes). Myrer et al30 have also recently published similar results. It is quite likely that we did not cool the tissues long enough to be able to determine if there was truly a difference between the different cold modalities at the deeper 2-cm subadipose depth. Potentially, this difference may even become visible with continued temperature measurement after removal of the cold modality following a 30-minute application because deep temperatures typically continue to fall for several minutes after application.1
CONCLUSIONS
We observed that cold modalities with different thermodynamic properties do, in fact, produce different IM temperatures during cryotherapy. However, it appears that some thermodynamic properties have larger effects than others. Among the most important of these is the effect of a change of physical state. Ice-based modalities go through a change of state from solid to liquid and as a result absorb substantially more heat. This increased heat absorption resulted in colder IM temperatures than were observed with gel packs. On the other hand, differences in thermal conduction and evaporation that might be found with a wet interface did not produce IM temperatures that were meaningfully different from typical ice bags. Therefore, working on the presently untested assumption that colder is better, we support the use of cold modalities that go through a change in physical state (ie, ice-based modalities) over other cold modalities in the management of musculoskeletal injuries.
REFERENCES
- 1.Merrick MA, Knight KL, Ingersoll CD, Potteiger JA. The effects of ice and compression wraps on intramuscular temperatures at various depths. J Athl Train. 1993;29:236–245. [PMC free article] [PubMed] [Google Scholar]
- 2.Merrick MA, Rankin JM, Andres FA, Hinman CL. A preliminary examination of cryotherapy and secondary injury in skeletal muscle. Med Sci Sports Exerc. 1999;31:1516–1520. doi: 10.1097/00005768-199911000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Knight KL. Cryotherapy in Sports Injury Management. Champaign, IL: Human Kinetics; 1995. [Google Scholar]
- 4.Knight KL. Effects of hypothermia on inflammation and swelling. Athl Train J Natl Athl Train Assoc. 1976;11:7–10. [Google Scholar]
- 5.Guyton AC. Textbook of Medical Physiology. 8th ed. Philadelphia, PA: WB Saunders; 1991. [Google Scholar]
- 6.Majno G, Joris I. Cells, Tissues, and Disease: Principles of General Pathology. Cambridge, MA: Blackwell Scientific; 1996. pp. 440–442. [Google Scholar]
- 7.Sapega AA, Heppenstall RB, Sokolow DP, et al. The bioenergetics of preservation of limbs before replantation: the rationale for immediate hypothermia. J Bone Joint Surg Am. 1988;70:1500–1513. [PubMed] [Google Scholar]
- 8.Abramson DI. Physiologic basis for the use of physical agents in peripheral vascular disorders. Arch Phys Med Rehabil. 1965;46:216–244. [PubMed] [Google Scholar]
- 9.Barcroft H, Edholm OG. The effect of temperature on blood flow and deep temperature in the human forearm. J Physiol. 1943;102:5–20. doi: 10.1113/jphysiol.1943.sp004009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Basset SW, Lake BM. Use of cold applications in the management of spasticity. Phys Ther Rev. 1958;38:333–334. doi: 10.1093/ptj/38.5.333. [DOI] [PubMed] [Google Scholar]
- 11.Johnson DJ, Moore S, Moore J, Oliver RA. Effects of cold submersion on intramuscular temperature of the gastrocnemius muscle. Phys Ther. 1979;59:1238–1242. doi: 10.1093/ptj/59.10.1238. [DOI] [PubMed] [Google Scholar]
- 12.Jutte LS, Merrick MA, Ingersoll CD, Edwards JE. The relationship between intramuscular temperature, skin temperature, and adipose thickness during cryotherapy and rewarming. Arch Phys Med Rehabil. 2001;82:845–850. doi: 10.1053/apmr.2001.23195. [DOI] [PubMed] [Google Scholar]
- 13.Knight KL, Aquino J, Johannes SM, Urban CD. A re-examination of Lewis' cold-induced vasodilatation in the finger and the ankle. Athl Train J Natl Athl Train Assoc. 1980;15:238–250. [Google Scholar]
- 14.Lowdon BJ, Moore RJ. Determinants and nature of intramuscular temperature changes during cold therapy. Am J Phys Med. 1975;54:223–233. [PubMed] [Google Scholar]
- 15.Mancuso DL, Knight KL. Effects of prior physical activity on skin surface temperature response of the ankle during and after a 30-minute ice pack application. J Athl Train. 1992;27:242–249. [PMC free article] [PubMed] [Google Scholar]
- 16.Meeusen R, Van der Veen P, Joos E, Roeykens J, Bossuyt A, De Meirleir K. The influence of cold and compression on lymph flow at the ankle. Clin J Sport Med. 1998;8:266–271. doi: 10.1097/00042752-199810000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Mlynarczyk JH. Skin Temperature Changes in the Ankle During and After Ice Pack Application of 10, 20, 30, 45, and 60 Minutes [master's thesis] Terre Haute, IN: Indiana State University; 1984. [Google Scholar]
- 18.Myrer JW, Measom G, Fellingham GW. Temperature changes in the human leg during and after two methods of cryotherapy. J Athl Train. 1998;33:25–29. [PMC free article] [PubMed] [Google Scholar]
- 19.Palmer JE, Knight KL. Ankle and thigh skin surface temperature changes with repeated ice pack application. J Athl Train. 1996;31:319–323. [PMC free article] [PubMed] [Google Scholar]
- 20.Varpalotai M, Knight KL. Pressures exerted by elastic wraps applied by beginning and advanced student athletic trainers to the ankle and the thigh with and without an ice pack. Athl Train J Natl Athl Train Assoc. 1991;26:246–250. [Google Scholar]
- 21.Columbus, OH: Columbus Instruments; 1987. Iso-Thermex-16 [package insert] [Google Scholar]
- 22.Favero M. Chemical disinfection of medical and surgical materials. In: Block SS, editor. Disinfection, Sterilization, and Preservation. 3rd ed. Philadelphia, PA: Lea & Febiger; 1983. pp. 469–486. [Google Scholar]
- 23.Curl WW, Smith BP, Marr A, Rosencrance E, Holden M, Smith TL. The effect of contusion and cryotherapy on skeletal muscle microcirculation. J Sports Med Phys Fitness. 1997;37:279–286. [PubMed] [Google Scholar]
- 24.Lide D, editor. CRC Handbook of Chemistry and Physics. 74th ed. Boca Raton, FL: CRC Press; 1994. pp. 5.1–5.17. [Google Scholar]
- 25.Halliday D, Resnick R. Fundamentals of Physics. 3rd ed. New York, NY: John Wiley & Sons; 1988. pp. 446–455. [Google Scholar]
- 26.Waylonis GW. The physiologic effects of ice massage. Arch Phys Med Rehabil. 1967;48:37–42. [PubMed] [Google Scholar]
- 27.McGown H. Effects of cold application on maximum isometric contraction. Phys Ther. 1967;47:185–192. doi: 10.1093/ptj/47.3.185. [DOI] [PubMed] [Google Scholar]
- 28.Hobbs K. Results of intramuscular temperature changes at various levels after the application of ice. Sports Health. 1983;1:15. [Google Scholar]
- 29.Otte JW, Merrick MA, Ingersoll CD, Cordova ML. Subcutaneous adipose tissue thickness changes cooling time during cryotherapy. Arch Phys Med Rehabil. 2002;83:1501–1505. doi: 10.1053/apmr.2002.34833. [DOI] [PubMed] [Google Scholar]
- 30.Myrer JW, Myrer KA, Measom GJ, Fellingham GW, Evers SL. Muscle temperature is affected by overlying adipose when cryotherapy is administered. J Athl Train. 2001;36:32–36. [PMC free article] [PubMed] [Google Scholar]