Abstract
Objective:
To examine the effects of oral creatine (Cr) monohydrate supplementation on muscle Cr concentration, body mass, and total body water (TBW), extracellular water (ECW), and intracellular water (ICW) volumes.
Design and Setting:
After an overnight fast, urinary Cr and creatinine concentrations, muscle Cr concentration, body mass, TBW, ECW, and ICW were measured, and subjects were randomly assigned to either a Cr or a placebo (P) group. The Cr group ingested 25 g/d of Cr for 7 days (loading phase) and 5 g/d for the remaining 21 days (maintenance phase), whereas the P group ingested a sucrose P using the same protocol. All the measures were reassessed immediately after the loading and maintenance phases.
Subjects:
Sixteen men (age = 22.8 ± 3.01 years, height = 179.8 ± 7.1 cm, body mass = 84.8 ± 11.2 kg) and 16 women (age = 21.8 ± 2.51 years, height = 163.4 ± 5.9 cm, body mass = 63.6 ± 14.0 kg) involved in resistance training volunteered to participate in this study.
Measurements:
Muscle Cr concentration was determined from the vastus lateralis muscle using a percutaneous needle-biopsy technique. Total body water, ECW, and ICW volumes were assessed using deuterium oxide and sodium bromide dilution analyses.
Results:
The Cr group experienced a significant increase in muscle Cr concentration, body mass, and TBW. The P group experienced a small but significant increase in TBW only.
Conclusions:
The Cr supplementation protocol was effective for increasing muscle Cr concentrations, body mass, and TBW; however, fluid distribution was not changed.
Keywords: ergogenic aids, hydration, fluid balance, body mass, body composition
Creatine (Cr) supplementation continues to be extremely popular as a potential ergogenic aid among athletes at all levels. Studies have shown that muscle Cr and phosphocreatine can be significantly elevated when a normal diet is supplemented with Cr.1–4 The theory behind its use is similar to that of carbohydrate loading, because an increased muscle Cr content would conceivably enhance the capacity of the phosphagen energy system, providing greater resistance to fatigue and improving performance. Anecdotal reports of ergogenic value have been supported by scientifically controlled studies investigating its effects on strength,5–8 power,8–11 speed,12 and fatigue.4,13,14 However, not all the findings support ergogenic claims.3,15,16
Numerous anecdotal reports have associated muscle cramping, spasm, strains, gastrointestinal distress, kidney dysfunction, and heat illness with Cr supplementation. At this time, however, the only side effect directly associated with Cr supplementation is weight gain.5–8,11,13,16–19 Some of these authors have reported increases in body mass after only a loading phase of supplementation (20–25 g/d for 5–7 days).8,11,13,16 Thus, it is likely that the gains are due more to greater water retention during supplementation than to lean-tissue accretion. It is conceivable that increased muscle Cr concentrations are associated with changes in the intracellular osmotic pressure, resulting in movement of water into the cell, water retention, and weight gain. When water loss through sweating occurs from exercise, increased environmental temperatures, or a combination of both, this intracellular fluid shift may be detrimental. For example, the water bound inside the cell may not be available to the extracellular compartment for thermal regulation. Therefore, cramping and other heat-related problems may result from a fluid shift occurring during supplementation. However, the existence of this Cr-related fluid shift is speculative because only 1 group19 to date has reported changes in fluid distribution after supplementation. It must be noted that fluid volumes were not measured directly in that study but were predicted using a bioelectric impedance analysis. Furthermore, muscle Cr was not assessed, so whether the changes in fluid distribution were actually associated with an increased muscle Cr concentration is unknown. Therefore, the purpose of this study was to investigate the effects of Cr supplementation on muscle Cr concentration and fluid distribution using a direct measure of fluid volumes.
METHODS
Subjects
Sixteen men (age = 22.8 ± 3.01 years, height = 179.8 ± 7.1 cm, body mass = 84.8 ± 11.2 kg) and 16 women (age = 21.8 ± 2.51 years, height = 163.4 ± 5.9 cm, body mass = 63.6 ± 14.0 kg) who were involved in a total-body resistance training program for at least 3 days per week were randomly assigned to either a Cr supplementation group or a placebo (P) group. We excluded subjects from participation if they had supplemented their habitual diet with any form of Cr during the past 60 days; were suffering from any form of kidney, liver, or endocrine disease or any disorder that might affect normal cellular levels of Cr or fluid balance (or both); or were taking any substance classified as a diuretic other than the caffeine found in their habitual diet. We also excluded women who were currently using oral contraceptives and women who had not completed 2 menstrual cycles since last using oral contraceptives. Before participating, each subject read a description of the study and signed an informed consent form approved by the university's institutional review board, which also approved the study.
Procedures
The evening before the supplementation period began, each subject reported to the university's General Clinical Research Center for an overnight stay and for baseline measurements. We instructed the subjects to refrain from any type of exercise after the last meal of the day before being admitted for the overnight stay. To control for changes in fluid distribution across the menstrual cycle, each woman began the study between days 1 and 7 of the cycle. Upon arrival at the Center, venous blood and urine samples were taken for a complete clinical chemistry panel (20 items) and for drugs of abuse, anabolic steroid, and pregnancy screen. After an overnight fast, we measured each subject for body mass, urinary Cr and creatinine concentrations, total body water (TBW) content, extracellular water (ECW) content, and muscle Cr concentration. Once these measurements were completed, the supplementation period began. We instructed the subjects to maintain their normal diets and their regular resistance-training programs throughout the entire supplementation and testing period. We also instructed the subjects to refrain from ingesting any type of nonsteroidal anti-inflammatory medication, nutritional supplement, or substance classified as a diuretic other than the caffeine found in the habitual diet. Each subject was required to maintain a training log in which the number of sets, repetitions, and the resistance used were recorded during each exercise session. Additionally, we asked each subject to complete a weekly questionnaire designed to determine the incidence of any adverse effects during supplementation. All subjects reported back to the Center on the evening of the seventh and 28th (final) day of supplementation, at which time the overnight fast and subsequent measurements were repeated.
Supplementation
We randomly assigned each subject to either a Cr supplementation group or a P group in a double-blind fashion. Subjects in the Cr group (8 men, 8 women) ingested 5.0 g of Phosphagen (Experimental and Applied Sciences, Inc, Golden, CO) mixed with 15 g of a flavored simple carbohydrate 5 times per day for 7 days (loading phase). Immediately after the loading phase, each subject ingested the same supplement once a day for the remaining 21 days (maintenance phase). Subjects in the P group (8 men, 8 women) ingested 20 g of the flavored simple carbohydrate for 28 days using the same protocol as the Cr group. Individual-dose packages, identical in weight, were prepared for the Cr and the P groups and dispensed weekly. We instructed the subjects to mix the powder supplement with 16 oz (0.47 L) of water and maintain a daily record of the supplementation times. Finally, to further monitor compliance, we asked each subject to return any unused portions at the end of each week.
Measurements
Urinary Creatine and Creatinine
Urine samples were collected and frozen at −80°C for later analysis of Cr and creatinine concentrations using high-performance liquid chromatography.
Muscle Creatine Concentration
After local anesthesia and incision of the skin, muscle samples were obtained from the vastus lateralis muscle of the nondominant leg using a percutaneous needle-biopsy technique modified to include suction.20 Immediately after removal, the tissue samples were frozen in liquid nitrogen and stored at −80°C for later analysis. When all testing sessions were completed, the tissue samples were packaged in dry ice and shipped overnight to a human performance laboratory, where they were later freeze dried, dissected free of any blood and connective tissue, and powdered. The powdered muscle was then extracted with perchloric acid, neutralized, and analyzed enzymatically for muscle Cr using a spectrophotometric analysis described by Harris et al.20
Body Mass and Total Body Water Volume
Body mass was assessed using a digital scale from the BOD POD Body Composition Tracking System (Life Measurements, Inc, Concord, CA). Total body water was determined using a deuterium oxide dilution method, which has been shown to be a valid and reliable measurement technique.21–24 We administered each subject an oral dose of deuterium oxide of approximately 0.15 g/kg of body mass, which was weighed out and diluted with sterile water for intake. Before ingestion and after a 4-hour equilibration period, two 5-mL venous blood samples were drawn for comparison. After extraction from the blood, the plasma was frozen at −20°C and stored for later analysis. Samples were then packaged with dry ice and shipped overnight to Metabolic Solutions, Inc (Nashua, NH), where deuterium enrichment in the body fluid was measured using isotope ratio mass spectrometry.22,24 The precision of this method is ±2%, and the percent coefficient of variation is typically 0.75% daily.21 Total body water was calculated as the deuterium-dilution space divided by 1.04, which corrects for exchange of the deuterium with nonaqueous hydrogen of body solids.21,22 We also assessed TBW using a Xitron 4200 multi-frequency bioelectrical impedance analyzer (Xitron Technologies Inc, San Diego, CA). This was performed as a backup measure in case we were unable to perform the dilution analysis on the plasma samples.
Intracellular Water and Extracellular Water Volumes
The ECW compartment was determined using a sodium bromide dilution method.25–27 We administered each subject an oral dose of sodium bromide (approximately 60 mg/kg of body mass) simultaneously with the deuterium oxide solution. The same blood samples taken for the deuterium analysis were also used for the sodium bromide analysis, with the bromide concentration in the serum ultrafiltrate determined by high-performance liquid chromatography (Metabolic Solutions, Inc). Because of the extreme sensitivity of this method, small quantities of bromide can be administered for the analysis, allowing for sufficient washout between measurement days.26 The corrected bromide space was calculated and used to determine the ECW volume.25–27 The intracellular water (ICW) volume was calculated by subtracting the ECW volume from the TBW volume.
Statistical Analysis
We used the Statistical Package for the Social Sciences software (version 10.0, SPSS Inc, Chicago, IL) to perform the statistical analysis of the raw data. All dependent measures were analyzed using a 1-within (time), 2-between (group and sex) mixed design with repeated-measures analyses of variance and Tukey Honestly Significant Difference post hoc tests to test differences between means. For all statistical tests, the alpha level was set at .05.
RESULTS
Blood and Urine Analyses
Analyses for anabolic steroids and drugs of abuse were negative for all subjects at each testing date, and no differences were observed in either group for blood or urine creatinine concentrations. However, the time-by-group (F2,56 = 25.56, P = .000) and time-by-sex (F2,56 = 4.04, P = .023) interactions for urinary Cr concentration were significant. The Cr group had significantly greater urinary Cr concentrations on days 7 and 28 as compared with presupplementation, whereas no changes were observed in the P group (Table). Furthermore, women experienced greater urinary Cr concentrations than did men on days 7 and 28.
Urinary and Muscle Creatine, Body Mass, Total Body Water, and Intracellular Water After Loading and Maintenance with Either Creatine or Placebo
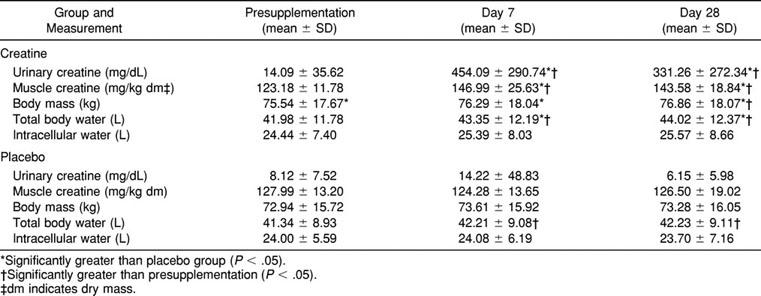
Muscle Creatine Concentration
Assays for Cr could not be completed for 4 subjects (2 each in the Cr and P groups); thus, these subjects were not included in the Cr analysis. The time-by-group interaction was significant (F2,48 = 4.75, P = .013): the Cr group had a greater Cr concentration on days 7 and 28 as compared with presupplementation (see Table). No changes were observed in the P group. No differences were observed when the men and women within the Cr group were compared before or after supplementation.
Body Mass
A significant time-by-group interaction (F2,56 = 3.30, P = .044) was observed for body mass, as the Cr group experienced a significant increase from presupplementation to day 28 (see Table). The Cr group's body mass on day 7 was 0.75 kg greater than at presupplementation; however, this change was not significant. The Tukey post hoc test revealed that an increase of 0.88 kg was necessary for statistical significance. The P group did not experience any significant changes in body mass. As expected, men had greater body mass than women, but there were no significant sex interactions.
Total Body Water Volume
A significant time-by-group interaction (F2,56 = 3.86, P = .027) was observed for TBW (see Table). Both groups experienced significantly greater TBW levels on days 7 and 28 as compared with presupplementation, but the increase was greater in the Cr group. The Tukey post hoc test revealed that a difference of 0.86 L was necessary for statistical significance. The differences of 0.87 and 0.89 L experienced by the P group were just higher than this value. Although no difference existed between the groups before supplementation, the Cr group had a significantly greater TBW volume on days 7 and 28 than did the P group. As expected, men had a greater TBW than women; however, there were no significant sex interactions.
Extracellular Water and Intracellular Water Volumes
As expected, men had greater ICW and ECW volumes, but there were no sex interactions. No other significant main effects or interactions, including a time-by-group interaction (F2,56 = 1.02, P = .366) for ICW, were observed. Furthermore, when ICW was expressed relative to the TBW, no significant changes were noted.
DISCUSSION
We examined the changes in muscle Cr, body mass, and body water during periods of Cr loading and maintenance in men and women. The supplementation protocol was indeed effective for increasing muscle Cr concentrations. The increases in muscle Cr were associated with increases in both TBW and body mass. However, only TBW increased during the loading phase, whereas the increase in body mass was not observed until both the loading and maintenance phases were completed.
Muscle Creatine
Several reports indicate that muscle Cr concentration can be elevated when a normal diet is supplemented with oral Cr.1–4 Our Cr group experienced a 20% increase in Cr concentration during the first week (loading phase) of supplementation. This increase was then maintained throughout the remaining 3 weeks (maintenance phase).
As expected, a large amount of between-subject variability was observed for the change in muscle Cr concentration from presupplementation to the end of the loading phase. Similar findings have been reported previously,1–3 which may explain why some individuals do not experience an ergogenic effect.15,16 Greenhaff28 reported that 20% to 30% of individuals do not respond to Cr supplementation (<10 mmol/kg dry mass [dm] [8%] in muscle Cr concentration). Similarly, we observed that 4 subjects (28%) in our study failed to respond to the supplementation. The dosages we used were not adjusted for body mass; thus, each subject ingested equal amounts of Cr. Because of this, it might be expected that a greater effect would be seen in individuals with smaller body mass. However, this was not observed because the nonresponders represented a wide range of body mass (57.09–86.21 kg). Furthermore, the increase experienced by women (body mass = 63.63 ± 12.58 kg) was not different from that experienced by men (body mass = 87.46 ± 11.98 kg). This is not unexpected because the 7-day loading protocol should have been sufficient for maximizing muscle uptake regardless of body mass.1 For example, women ingesting Cr experienced a greater increase in urinary Cr excretion than did men after both the loading and maintenance phases. Although sex differences have not been reported previously, they are not unexpected. Any Cr not taken in by the muscle or other tissues remains in the plasma and is eventually filtered through the kidneys and excreted in the urine. Because of their lower body mass, women had less available tissue for Cr uptake, resulting in a greater amount of excess Cr. Thus, factors other than the relative dose-response relationship play a role in the total Cr uptake by the muscle.
Greenhaff28 observed that 20% of individuals achieved Cr concentrations of approximately 150 to 160 mmol/kg dm after supplementation. This concentration is considered to be the upper limit of muscle Cr stores, although some individuals, including 4 subjects in the present study, achieve higher levels. Whereas a number of factors have been suggested to affect muscle Cr uptake, the primary determinant appears to be the initial muscle Cr concentration.1,3,28 The normal muscle Cr concentration of the human vastus lateralis appears to be approximately 124 mmol/kg dm.2,20 In our subjects, the average muscle Cr concentration before supplementation was 125.6 mmol/kg dm, with a large amount of between-subject variability (105.9–150.8 mmol/kg dm). Our findings are consistent with those of previous authors,1–3 who reported a wide variation (100–150 mmol/kg dm) in the initial muscle Cr content. Increases in muscle Cr concentration after supplementation appear to be inversely related to the initial Cr concentration.1,28
In support of the above, we observed a significant correlation between the initial Cr levels and the increases in muscle Cr after supplementation (r = −0.75, P < .01). Subjects in the Cr group with an initial Cr concentration less than 120 mmol/kg dm experienced an average increase of 33.4 mmol/kg dm (23.1%), whereas subjects with an initial Cr concentration greater than 120 mmol/kg dm only experienced an average increase of 16.6 mmol/kg dm (11.2%) during the loading phase.
Body Mass
Previous investigators8,11,13,16 have reported increases in body mass after a loading phase of Cr supplementation. Although the Cr group did experience an increase in the present study, the increase did not reach significance until the entire supplementation protocol had been completed (1.31-kg increase after 28 days). In contrast, the P group failed to experience any significant changes in body mass. This is consistent with other studies reporting increases after a protocol of Cr loading and maintenance.6,7,17–19 However, body mass measurements were not taken immediately after the loading phase in those studies. Thus, whether their subjects experienced any immediate changes in body mass is unknown. In our study, the increase observed in the Cr group after the loading phase (0.75 kg) was slightly lower than what the Tukey post hoc test demonstrated as necessary for significance (0.88 kg). This was unexpected because increases of 0.70 and 0.75 kg after loading had previously been found to be statistically significant.11,16 However, our findings are not isolated because others12,14,29,30 have also failed to observe significant body mass changes after a loading phase of supplementation.
Although 4 subjects (including 3 women) in the Cr group failed to experience an increase in body mass after 28 days of supplementation, the range for those who did was consistent with gains previously observed, from 0.47 to 3.92 kg.6,7,17–19 At this time, the exact cause of the weight gain has not been determined. However, increases in protein synthesis and water retention are the 2 more commonly cited theories.
Protein Synthesis
It has been theorized that at least part of the increase in body mass can be attributed to increased protein synthesis and morphologic changes within the skeletal muscle.13 Recently reported data suggest that Cr supplementation might amplify protein synthesis stimulation in response to resistance training.31 In that study, however, protein content was only examined after 12 weeks of supplementation and training. Thus, the effect of Cr after only 1 week or even after 4 weeks of training is unknown. As mentioned previously, because a number of authors8,11,13,16 reported increases in body mass in as few as 5 to 7 days, it is unlikely that these changes can be explained by protein synthesis alone.
Water Retention
The gains in body mass observed are likely due to water retention during supplementation. Creatine is an osmotically active substance. Thus, any increase in the body's Cr content should result in increased water retention and consequent gains in body mass.2,8 For example, 1 subject in the present study, who reported having a fairly consistent body mass throughout the previous year, experienced a 4.8-kg increase in body mass during the first week of supplementation, 90% of which was accounted for by the increase in TBW. The limited number of investigators12,17–19,32 who have reported TBW volumes during supplementation provide contrasting results. Significant increases in TBW have been observed after 619 and 917 weeks of supplementation, whereas a similar 4-week protocol failed to affect TBW.32 However, whether the subjects in these studies actually experienced an increase in muscle Cr is unknown because Cr concentrations were not measured. Furthermore, in each of these studies, a bioelectric impedance analysis was used to estimate TBW. Such analysis is a prediction of TBW and is associated with errors ranging from 1.5 to 2.5 L.33 Dilution techniques, such as the deuterium oxide and sodium bromide we used, are considered criterion measures of body water.34,35 To our knowledge, we are the first to directly measure muscle Cr and fluid balance during supplementation, and our results suggest that increases in muscle Cr concentrations are associated with water retention. Both groups had similar TBW volumes at the beginning of the study. However, although both groups experienced an increase in TBW during the first week of supplementation, the Cr group's TBW was significantly greater than the P group's TBW after both the loading and maintenance phases (see Table). These findings support previous research because the increase experienced by the Cr group (4.86%) is similar to that observed using the bioelectric impedance analysis measure (5.30% and 4.47%).17,19
We initially theorized that water retention would be responsible for the immediate gains in body mass after supplementation. As expected, the changes in TBW experienced by the Cr group were similar to those observed for body mass. The greatest increase in TBW (1.37 L) occurred during the first week of supplementation, whereas the greatest difference compared with presupplementation occurred after 28 days of supplementation (2.04 L). It is interesting to note, however, that the increase in TBW we observed would actually account for a greater increase in body mass. We would expect that a TBW increase of 1.37 L would be associated with a body mass increase of approximately 1.37 kg. Yet, the Cr group only experienced a nonsignificant 0.75-kg increase during this time. An explanation for this finding is not readily apparent but must involve decreased caloric intake or increased caloric expenditure (or both) during supplementation. A BOD POD Body Composition Tracking System digital scale was used for all body mass measurements, and a dilution technique was used to determine TBW. We also performed a bioelectric impedance analysis assessment of TBW as a secondary measure. When we analyzed these data, we observed a significant and identical 1.37-L increase in TBW after the loading phase. Thus, we do not question the validity of our data.
Fluid Intake
During the loading phase, the subjects in both groups ingested their respective supplements with approximately 454 mL of water 5 times per day. It is possible that the increase in TBW content is the result of an increased fluid intake during the week (∼15.89 L). This is the most likely explanation for the slight increase experienced by the P group. However, the greater increase experienced by the Cr group suggests that the subjects experienced greater water retention. Thus, Cr uptake may have influenced water retention. Because fluid intake and urinary volume were not assessed, we could not determine whether increased fluid intake or decreased fluid loss or both were responsible for the change in body water.
Caloric Intake and Expenditure
We instructed our subjects to maintain their normal, habitual diet throughout the supplementation period. Although we did not record dietary intake, other authors8,10,18 have reported consistent caloric intakes while increases in body mass occurred during supplementation. Anecdotally, our subjects reported a decreased appetite at times during the supplementation period because they described a “full” feeling from ingesting the supplement and fluid on 5 occasions each day. Decreases in caloric intake during supplementation have been previously reported.6,7 Unfortunately, TBW volumes were not assessed in those studies; thus, it is unknown if they increased or not. It is also possible that the subjects in the Cr group experienced an ergogenic effect, allowing for greater training volume during this period. This may have resulted in greater caloric expenditure. Thus, it is possible that increased fluid intake, decreased caloric intake, and increased caloric expenditure are responsible for our findings.
Intracellular Water Retention
Because Cr is primarily stored intramuscularly (95%), it is more likely that the increase in TBW would be intracellular because of the direct influx of water into the muscle cell. Previous investigators12,18,32 were unable to determine whether increases in lean body mass were due to cellular water retention or gains in actual muscle protein, because only TBW was assessed during supplementation. More recently, however, fluid distribution has been assessed but only using the bioelectric impedance analysis prediction.17,19 We directly measured fluid distribution using dilution techniques and observed a 1.13-L (4.62%) increase in ICW in the Cr group. However, this change was not statistically significant. Increases in ICW have been reported previously using the bioelectric impedance analysis prediction.17,19 In those studies, increases of 3.30 L (9.0%) and 1.00 L (4.93%) were statistically significant.
The nonsignificant increase we observed accounted for 55.4% of the increase in TBW volume, which is fairly consistent with normal fluid distribution (approximately two thirds of the TBW is intracellular). This suggests an equal distribution of fluid during supplementation. Interestingly, an increase in cell volume appears to be an anabolic proliferative signal, which may be the first step in muscle protein synthesis.36,37 Because of this, increased cell volume has been suggested as a mechanism for protein synthesis stimulation and increased muscle mass under conditions of muscular overload during Cr supplementation.7,8
Adverse Effects
None of the blood or urinary measures suggested any adverse effects as a result of supplementation. Furthermore, none of the subjects in the present investigation experienced muscle cramping or any other side effects (other than increased body mass) during the supplementation protocol. However, whether any subjects performed exercise bouts resulting in a large amount of water loss is unknown. Thus, a potential relationship between Cr supplementation and heat illness cannot be established from the results of the present investigation.
Limitations of the Study
As mentioned previously, caloric and fluid intakes were not recorded, and differences in either of these factors could have influenced the changes in body mass and fluid balance. Furthermore, each subject was involved in an individualized resistance training program. These training protocols were not controlled for; however, the volume of each training session was recorded. Because of a large amount of variability across subjects, a relationship between training volume and changes in body mass and fluid balance could not be established. Furthermore, training protocols before supplementation were not recorded, so comparisons could not be made. Thus, it is possible that the differences in training volume may also have influenced the changes in body mass and fluid balance.6,38,39
CONCLUSIONS
Our results indicate that the supplementation protocol was effective in increasing muscle Cr concentrations. Increased muscle Cr content was associated with an increased body mass and TBW volume. Thus, supplementation does result in water retention. It was initially hypothesized that the water would be preferentially retained intracellularly, altering fluid distribution. However, this was not observed. Therefore, the theory of a Cr-related fluid shift is not supported because fluid distribution remained normal. An alteration in fluid distribution during supplementation had been suggested as a cause of muscle cramping and other heat-related problems anecdotally associated with Cr supplementation. Because the subjects failed to experience any side effects beyond weight gain, it cannot be determined whether athletes supplementing their habitual diet with oral Cr monohydrate will be more predisposed to muscle cramping and heat illness than athletes who are not ingesting Cr. However, our results do not support the fluid-shift theory behind Cr supplementation and heat illness. Thus, from this investigation, a potential relationship between Cr supplementation and heat illness cannot be established. It will be of value for future researchers to focus on changes in fluid balance and the occurrence of heat illness when Cr supplementation is combined with fluid loss during exercise.
ACKNOWLEDGMENTS
This work was supported in part by a grant from the National Athletic Trainers' Association Research and Education Foundation and in part by a grant from the National Institutes of Health to the University of Virginia, General Clinical Research Center, MO1 RR00847. We thank Dr Lori Wideman, Dr Judy Weltman, Sandra Jackson, RN, and the University of Virginia General Clinical Research Center staff for their assistance during the data collection. We also thank Experimental and Applied Sciences (EAS) Inc, Golden, CO, for supplying the Cr monohydrate used in the investigation.
REFERENCES
- 1.Harris RC, Soderlund K, Hultman E. Elevation of creatine in resting and exercised muscle of normal subjects by creatine supplementation. Clin Sci (Lond) 1992;83:367–374. doi: 10.1042/cs0830367. [DOI] [PubMed] [Google Scholar]
- 2.Hultman E, Soderlund K, Timmons JA, Cederblad G, Greenhaff PL. Muscle creatine loading in men. J Appl Physiol. 1996;81:231–237. doi: 10.1152/jappl.1996.81.1.232. [DOI] [PubMed] [Google Scholar]
- 3.McKenna MJ, Morton J, Selig SE, Snow RJ. Creatine supplementation increases muscle total creatine but not maximal intermittent exercise performance. J Appl Physiol. 1999;87:2244–2252. doi: 10.1152/jappl.1999.87.6.2244. [DOI] [PubMed] [Google Scholar]
- 4.Preen D, Dawson B, Goodman C, Lawrence S, Beilby J, Ching S. Effect of creatine loading on long term sprint exercise performance and metabolism. Med Sci Sports Exerc. 2001;33:814–821. doi: 10.1097/00005768-200105000-00022. [DOI] [PubMed] [Google Scholar]
- 5.Becque MD, Lochmann JD, Melrose DR. Effects of oral creatine supplementation on muscular strength and body composition. Med Sci Sports Exerc. 2000;31:654–658. doi: 10.1097/00005768-200003000-00016. [DOI] [PubMed] [Google Scholar]
- 6.Earnest CP, Snell PG, Rodriguez R, Almada AL, Mitchell TL. The effect of creatine monohydrate ingestion on anaerobic power indices, muscular strength and body composition. Acta Physiol Scand. 1995;153:207–209. doi: 10.1111/j.1748-1716.1995.tb09854.x. [DOI] [PubMed] [Google Scholar]
- 7.Kelly VG, Jenkins DG. Effect of oral creatine supplementation on near-maximal strength and repeated sets of high-intensity bench press exercise. J Strength Cond Res. 1998;12:109–115. [Google Scholar]
- 8.Volek JS, Kraemer WJ, Bush JA, et al. Creatine supplementation enhances muscular performance during high intensity resistance exercise. J Am Diet Assoc. 1997;97:765–770. doi: 10.1016/S0002-8223(97)00189-2. [DOI] [PubMed] [Google Scholar]
- 9.Hafe GG, Kirksey KB, Stone MH. The effect of 6 weeks of creatine monohydrate supplementation on dynamic rate of force development. J Strength Cond Res. 2000;14:426–433. [Google Scholar]
- 10.Kirksey B, Stone MH, Warren BJ, et al. The effects of 6 weeks of creatine monohydrate supplementation on performance measures and body composition in collegiate track and field athletes. J Strength Cond Res. 1999;13:148–156. [Google Scholar]
- 11.Volek JS, Mazzetti SA, Farquhar WB, Barnes BR, Gomez AL, Kraemer WJ. Physiological responses to short-term exercise in the heat after creatine loading. Med Sci Sports Exerc. 2001;33:1101–1108. doi: 10.1097/00005768-200107000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Grindstaff PD, Kreider R, Bishop R, et al. Effects of creatine supplementation on repetitive sprint performance and body composition in competitive swimmers. Int J Sport Nutr. 1997;7:330–346. doi: 10.1123/ijsn.7.4.330. [DOI] [PubMed] [Google Scholar]
- 13.Balsom PD, Ekblom B, Soderlund K, Sjodin B, Hultman E. Creatine supplementation and dynamic high-intensity intermittent exercise. Scand J Med Sci Sports. 1993;3:143–149. [Google Scholar]
- 14.Stout J, Eckerson J, Ebersole K, et al. Effect of creatine loading on neuromuscular fatigue threshold. J Appl Physiol. 2000;88:109–112. doi: 10.1152/jappl.2000.88.1.109. [DOI] [PubMed] [Google Scholar]
- 15.Gilliam JD, Hohzorn C, Martin D, Trimble MH. Effect of oral creatine supplementation on isokinetic torque production. Med Sci Sports Exerc. 2000;31:993–996. doi: 10.1097/00005768-200005000-00017. [DOI] [PubMed] [Google Scholar]
- 16.Mujika I, Chatard JC, Lacoste L, Barale F, Geyssant A. Creatine supplementation does not improve sprint performance in competitive swimmers. Med Sci Sports Exerc. 1996;28:1435–1441. doi: 10.1097/00005768-199611000-00014. [DOI] [PubMed] [Google Scholar]
- 17.Bemben MG, Bemben DA, Loftiss DD, Knehans AW. Creatine supplementation during resistance training in college football athletes. Med Sci Sports Exerc. 2001;33:1667–1673. doi: 10.1097/00005768-200110000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Kreider RB, Ferreira M, Wilson M, et al. Effects of creatine supplementation on body composition, strength, and sprint performance. Med Sci Sports Exerc. 1998;30:73–82. doi: 10.1097/00005768-199801000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Francaux M, Poortman JR. Effects of training and creatine supplement on muscle strength and body mass. Eur J Appl Physiol. 1999;80:165–168. doi: 10.1007/s004210050575. [DOI] [PubMed] [Google Scholar]
- 20.Harris RC, Hultman E, Nordesjo LO. Glycogen, glycolytic intermediates and high energy phosphates determined in biopsy samples of muscular quadriceps femoris of man at rest: methods and variance of values. Scand J Clin Lab Invest. 1974;33:109–120. [PubMed] [Google Scholar]
- 21.Measurement of body composition using deuterium oxide. Technical Paper 913. Nashua, NH: Metabolic Solutions Inc; 1999. [Google Scholar]
- 22.Evans EM, Saunders MJ, Spano MA, Arngrimsson SA, Lewis RD, Cureton KJ. Body composition changes with diet and exercise in obese women: a comparison of estimates from clinical methods and a 4-component model. Am J Clin Nutr. 1999;70:5–12. doi: 10.1093/ajcn/70.1.5. [DOI] [PubMed] [Google Scholar]
- 23.Lukaski HC, Johnson PE. A simple, inexpensive method of determining total body water using a tracer dose of D2O and infrared absorption of biological fluids. Am J Clin Nutr. 1985;41:363–370. doi: 10.1093/ajcn/41.2.363. [DOI] [PubMed] [Google Scholar]
- 24.Penman AD, Wright IA. Determination of deuterium level in biological fluids by isotope ratio mass spectrometry. Biomed Environ Mass Spectrom. 1987;14:339–342. doi: 10.1002/bms.1200140708. [DOI] [PubMed] [Google Scholar]
- 25.Extracellular water measurements with sodium bromide. Technical Paper 920. Nashua, NH: Metabolic Solutions Inc; 1996. [Google Scholar]
- 26.Miller ME, Cosgriff JM, Forbes GB. Bromide space determination using anion-exchange chromatography for measurement of bromide. Am J Clin Nutr. 1989;50:168–171. doi: 10.1093/ajcn/50.1.168. [DOI] [PubMed] [Google Scholar]
- 27.van Marken Lichtenbelt WD, Snel YEM, Brummer RJM, Koppeschaar HPF. Deuterium and bromide dilution, and bioimpedance spectrometry independently show that growth hormone-deficient adults have an enlarged extracellular water compartment related to intracellular water. J Clin Endocrinol Metab. 1997;82:907–911. doi: 10.1210/jcem.82.3.3833. [DOI] [PubMed] [Google Scholar]
- 28.Greenhaff PL. Creatine supplementation: recent developments. Br J Sports Med. 1996;30:276–277. doi: 10.1136/bjsm.30.4.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barnett C, Hinds M, Jenkins DG. Effects of oral creatine supplementation on multiple sprint cycle performance. Aust J Sci Med Sport. 1996;28:35–39. [PubMed] [Google Scholar]
- 30.Stout JR, Eckerson JM, Housh TJ, Ebersole KT. The effects of creatine supplementation on anaerobic working capacity. J Strength Cond Res. 1999;13:135–138. [Google Scholar]
- 31.Willoughby DS, Rosene J. Effects of oral creatine and resistance training on myosin heavy chain expression. Med Sci Sports Exerc. 2001;33:1674–1681. doi: 10.1097/00005768-200110000-00010. [DOI] [PubMed] [Google Scholar]
- 32.Kreider RB, Klesges R, Harmon K, et al. Effects of ingesting supplements designed to promote lean tissue accretion on body composition during resistance exercise. Int J Sport Nutr. 1996;6:234–246. doi: 10.1123/ijsn.6.3.234. [DOI] [PubMed] [Google Scholar]
- 33.Baumgartner RN. Electrical impedance and total body electrical conductivity. In: Roche A, editor. Human Body Composition. Champaign, IL: Human Kinetics; 1996. pp. 79–94. [Google Scholar]
- 34.Schoeller DA. Hydrometry. In: Roche A, editor. Human Body Composition. Champaign, IL: Human Kinetics; 1996. pp. 25–43. [Google Scholar]
- 35.Armstrong LE, Kenefick RW, Castellani JW, et al. Bioimpedance spectroscopy technique: intra-, extracellular, and total body water. Med Sci Sports Exerc. 1997;29:1657–1663. doi: 10.1097/00005768-199712000-00017. [DOI] [PubMed] [Google Scholar]
- 36.Haussinger D, Lang F, Gerok W. Regulation of cell function by the cellular hydration state. Am J Physiol. 1994;267(3 Pt 1):E343–E355. doi: 10.1152/ajpendo.1994.267.3.E343. [DOI] [PubMed] [Google Scholar]
- 37.Haussinger D. The role of cell hydration in the regulation of cell function. Biochem J. 1996;313(Pt 3):697–710. doi: 10.1042/bj3130697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Modlesky CM, Cureton KJ, Lewis RD, Prior BM, Sloniger MA, Rowe DA. Density of the fat-free mass and estimates of body composition in male weight trainers. J Appl Physiol. 1996;80:2085–2096. doi: 10.1152/jappl.1996.80.6.2085. [DOI] [PubMed] [Google Scholar]
- 39.Campbell WW, Crim MC, Young VR, Evans WJ. Increased energy requirements and changes in body composition with resistance training in older adults. Am J Clin Nutr. 1994;60:167–175. doi: 10.1093/ajcn/60.2.167. [DOI] [PubMed] [Google Scholar]


