Abstract
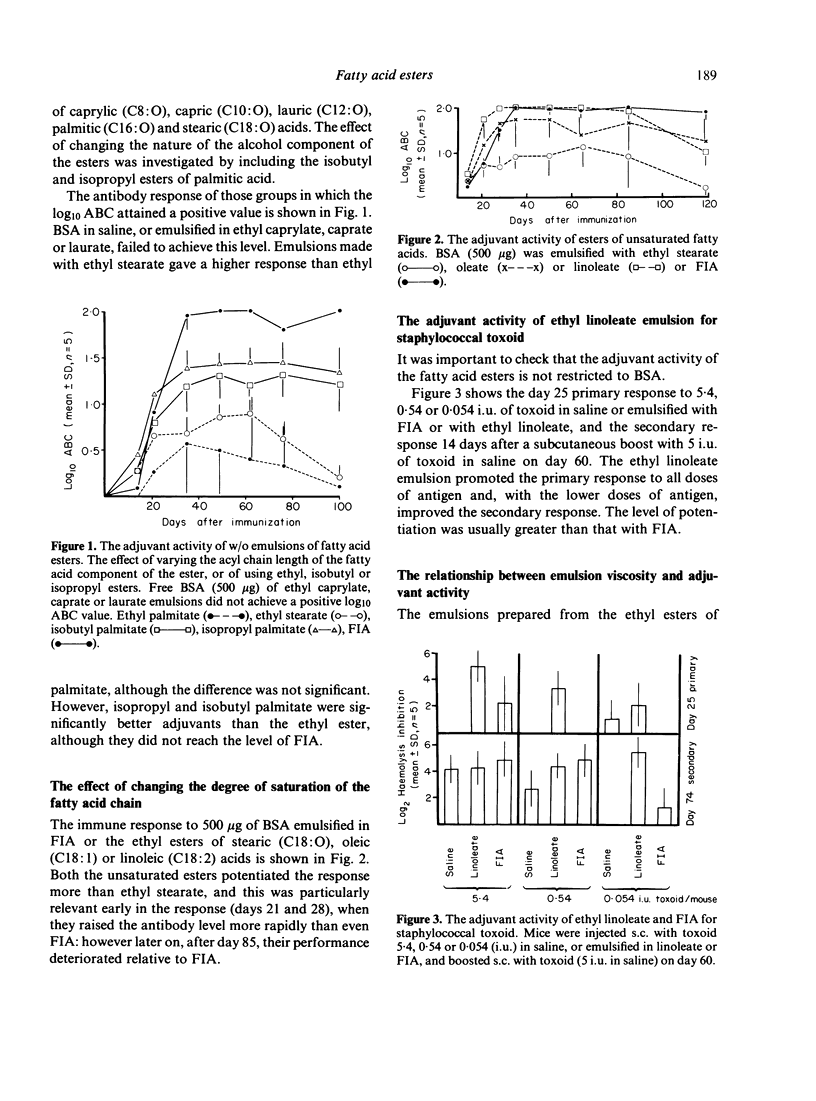
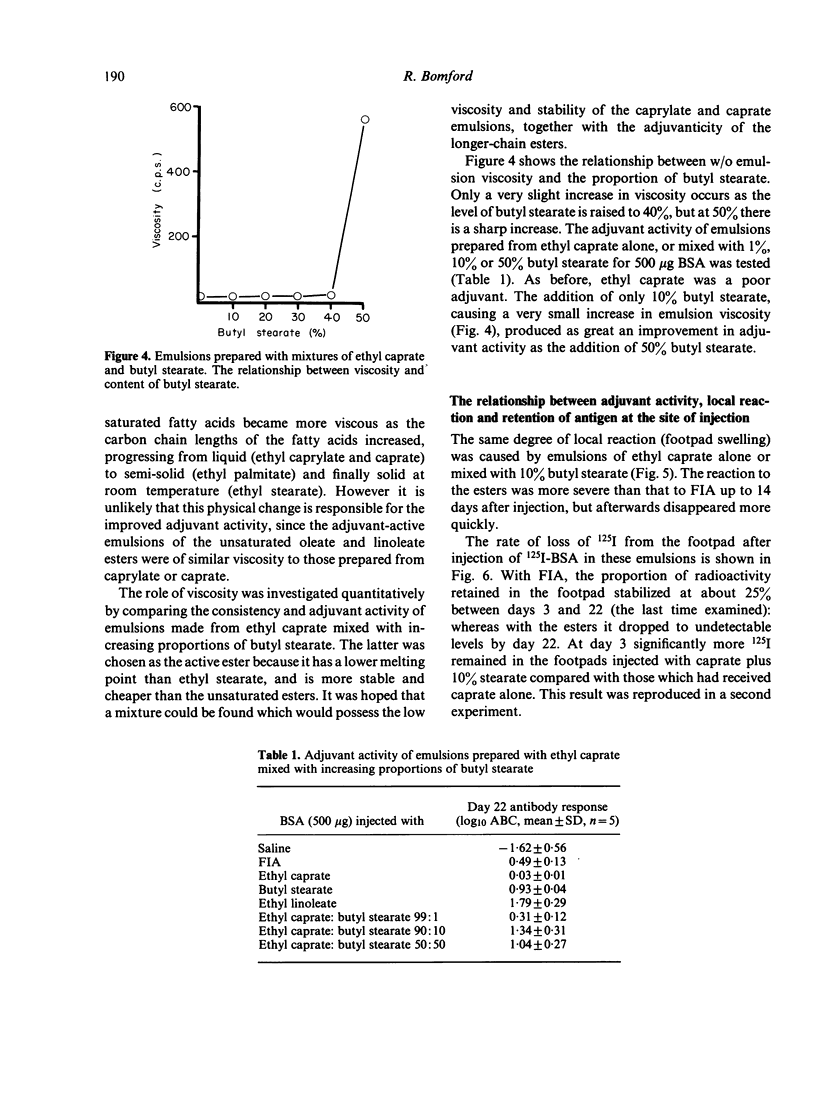
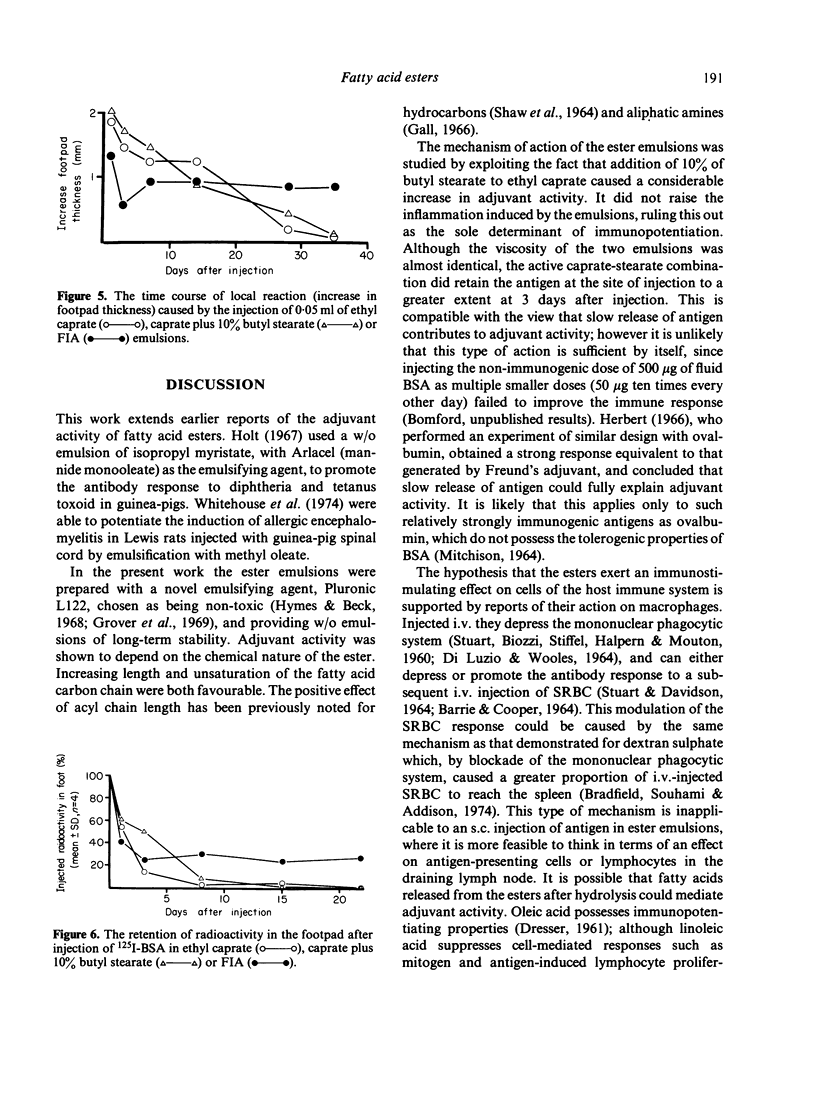
Water-in-oil emulsions of metabolizable fatty acid esters, with the non-toxic surfactant Pluronic L122 as emulsifying agent, potentiated the humoral response to bovine serum albumin and staphylococcal toxoid in the mouse. Adjuvant activity was increased by changing the chemical nature of the esters as follows: (i) using a series of ethyl esters, adjuvant activity appeared when the acyl chain length of the fatty acid component was 16 or greater; (ii) isobutyl and isopropyl esters of palmitic acid (C16:0) were superior to ethyl; (iii) the ethyl esters of oleic (C18:1) and linoleic (C18:2) acids were better than stearic (C18:0). Since emulsions prepared with longer chain saturated esters are very viscous or solid at room temperature, and unsaturated esters are chemically reactive, emulsions were prepared with differing proportions of ethyl caprate (C10:0) and butyl stearate. At a ratio of 9:1 the emulsions possessed the low viscosity of ethyl caprate, but gained the adjuvant activity of butyl stearate. 125I-labelled BSA was retained in the footpad to a significantly greater extent than with a caprate emulsion, but reasons are given for believing that slow release of antigen is not the only mechanism of adjuvant activity. The ester emulsions caused more acute but less chronic local inflammation (footpad swelling) than Freund's incomplete adjuvant.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARRIE J. U., COOPER G. N. ENHANCEMENT OF THE PRIMARY ANTIBODY RESPONSE TO PARTICULATE ANTIGENS BY SIMPLE LIPIDS. J Immunol. 1964 Apr;92:529–539. [PubMed] [Google Scholar]
- Bell E. B., Shand F. L. Cellular events in protein tolerant inbred rats. I. The fate of thoracic duct lymphocytes and memory cells during tolerance induction to human serum albumin. Eur J Immunol. 1973 May;3(5):259–267. doi: 10.1002/eji.1830030503. [DOI] [PubMed] [Google Scholar]
- Bradfield J. W., Souhami R. L., Addison I. E. The mechanism of the adjuvant effect of dextran sulphate. Immunology. 1974 Feb;26(2):383–392. [PMC free article] [PubMed] [Google Scholar]
- Cassidy P., Harshman S. Purification of staphylococcal alpha-toxin by adsorption chromatography on glass. Infect Immun. 1976 Mar;13(3):982–986. doi: 10.1128/iai.13.3.982-986.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DILUZIO N. R., WOOLES W. R. DEPRESSION OF PHAGOCYTIC ACTIVITY AND IMMUNE RESPONSE BY METHYL PALMITATE. Am J Physiol. 1964 May;206:939–943. doi: 10.1152/ajplegacy.1964.206.5.939. [DOI] [PubMed] [Google Scholar]
- DRESSER D. W. Effectiveness of lipid and lipidophilic substances as adjuvants. Nature. 1961 Sep 16;191:1169–1171. doi: 10.1038/1911169a0. [DOI] [PubMed] [Google Scholar]
- FARR R. S. A quantitative immunochemical measure of the primary interaction between I BSA and antibody. J Infect Dis. 1958 Nov-Dec;103(3):239–262. doi: 10.1093/infdis/103.3.239. [DOI] [PubMed] [Google Scholar]
- FREUND J. The mode of action of immunologic adjuvants. Bibl Tuberc. 1956;(10):130–148. [PubMed] [Google Scholar]
- Gall D. The adjuvant activity of aliphatic nitrogenous bases. Immunology. 1966 Oct;11(4):369–386. [PMC free article] [PubMed] [Google Scholar]
- Herbert W. J. Antigenicity of soluble protein in the presence of high levels of antibody: a possible mode of action of the antigen adjuvants. Nature. 1966 May 14;210(5037):747–748. doi: 10.1038/210747a0. [DOI] [PubMed] [Google Scholar]
- Hilleman M. R. Critical appraisal of emulsified oil adjuvants applied to viral vaccines. Prog Med Virol. 1966;8:131–182. [PubMed] [Google Scholar]
- Hymes A. C., Beck K. A comparison of pluronic F-68, low molecular weight dextran, mannitol, and saline as priming agents in the heart-lung apparatus. II. The in vitro influence on oxygen consumption of certain fluids used as priming agents in a heart-lung bypass apparatus. J Thorac Cardiovasc Surg. 1968 Jul;56(1):23–27. [PubMed] [Google Scholar]
- MITCHISON N. A. INDUCTION OF IMMUNOLOGICAL PARALYSIS IN TWO ZONES OF DOSAGE. Proc R Soc Lond B Biol Sci. 1964 Dec 15;161:275–292. doi: 10.1098/rspb.1964.0093. [DOI] [PubMed] [Google Scholar]
- Mertin J., Hughes D. Specific inhibitory action of polyunsaturated fatty acids on lymphocyte transformation induced by PHA and PPD. Int Arch Allergy Appl Immunol. 1975;48(2):203–210. doi: 10.1159/000231306. [DOI] [PubMed] [Google Scholar]
- Reynolds J. A., Harrington D. G., Crabbs C. L., Peters C. J., Di Luzio N. R. Adjuvant activity of a novel metabolizable lipid emulsion with inactivated viral vaccines. Infect Immun. 1980 Jun;28(3):937–943. doi: 10.1128/iai.28.3.937-943.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHAW C. M., ALVORD E. C., Jr, KIES M. W. STRAIGHT CHAIN HYDROCARBONS AS SUBSTITUTES FOR THE OIL IN FREUND'S ADJUVANTS IN THE PRODUCTION OF EXPERIMENTAL "ALLERGIC" ENCEPHALOMYELITIS IN THE GUINEA PIG. J Immunol. 1964 Jan;92:24–27. [PubMed] [Google Scholar]
- STUART A. E., DAVIDSON A. E. EFFECT OF SIMPLE LIPIDS ON ANTIBODY FORMATION AFTER INJECTION OF FOREIGN RED CELLS. J Pathol Bacteriol. 1964 Apr;87:305–316. doi: 10.1002/path.1700870210. [DOI] [PubMed] [Google Scholar]
- WILNER B. I., EVERS M. A., TROUTMAN H. D., TRADER F. W., MCLEAN I. W. VACCINE POTENTIATION BY EMULSIFICATION WITH PURE HYDROCARBON COMPOUNDS. J Immunol. 1963 Aug;91:210–229. [PubMed] [Google Scholar]
- Whitehouse M. W., Orr K. J., Beck F. W., Pearson C. M. Freund's adjuvants: relationship of arthritogenicity and adjuvanticity in rats to vehicle composition. Immunology. 1974 Aug;27(2):311–330. [PMC free article] [PubMed] [Google Scholar]


