Abstract
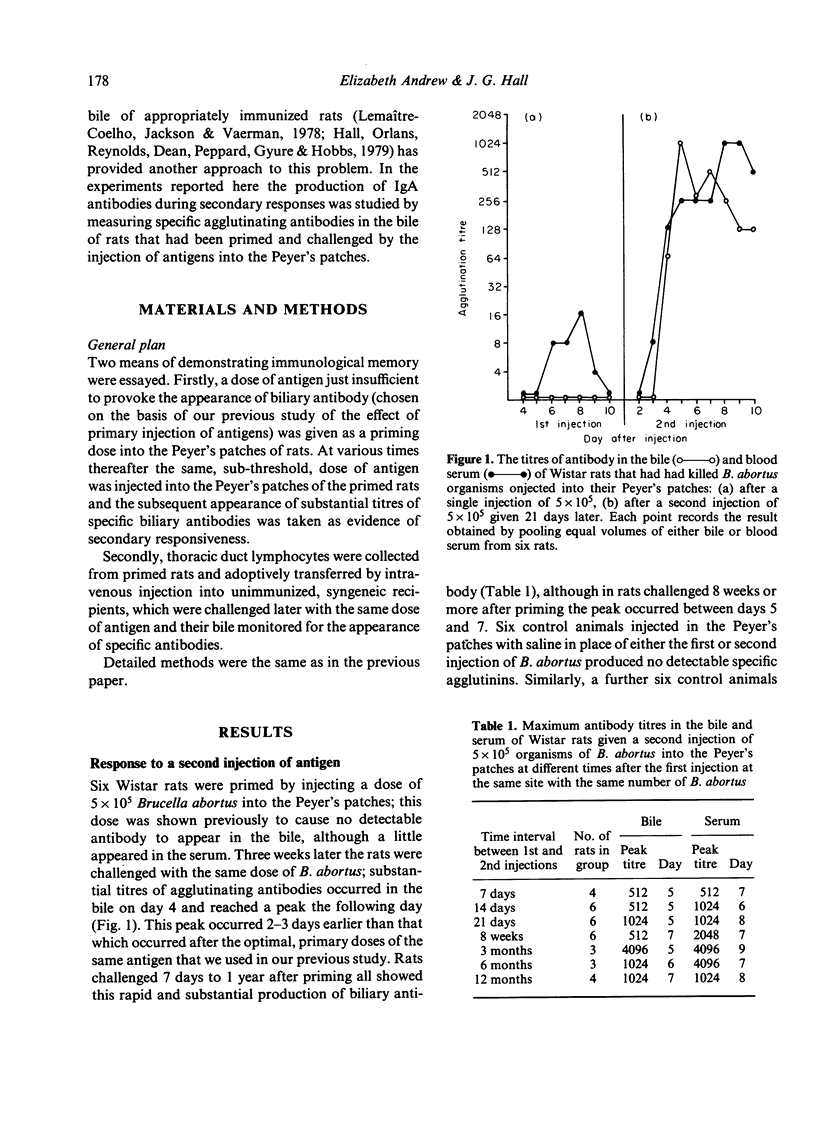
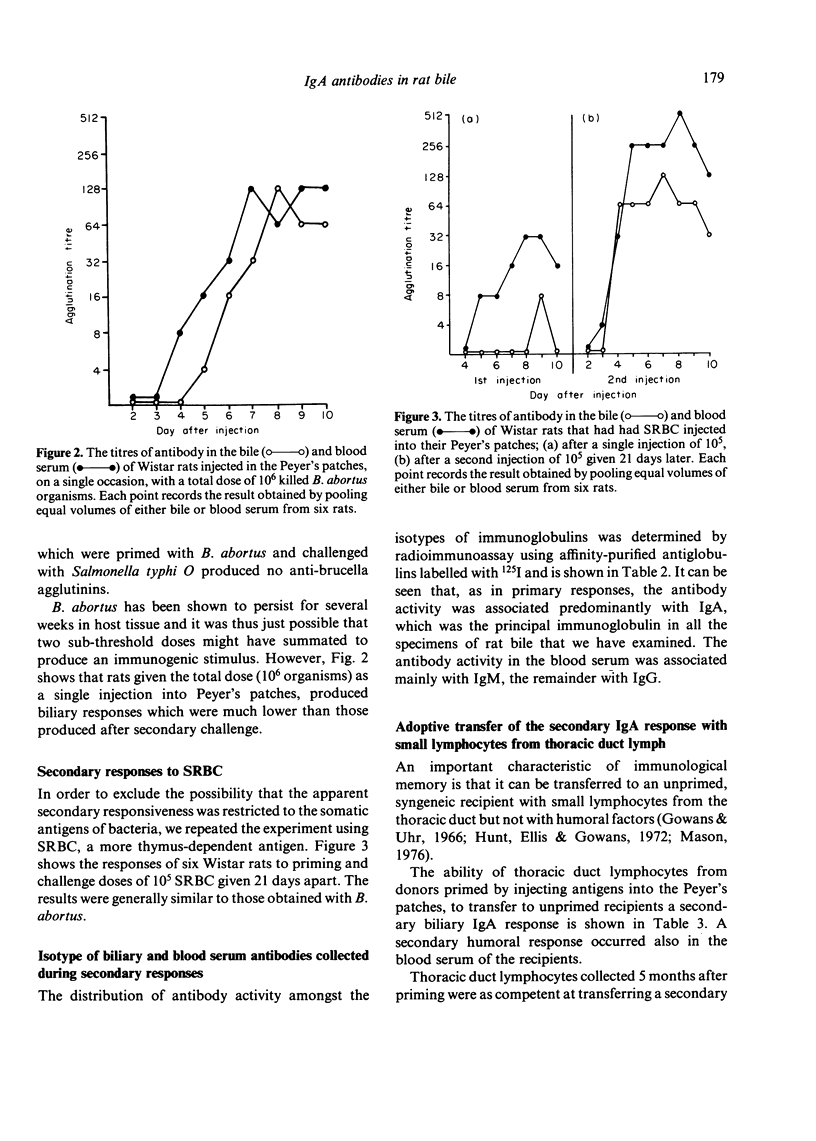
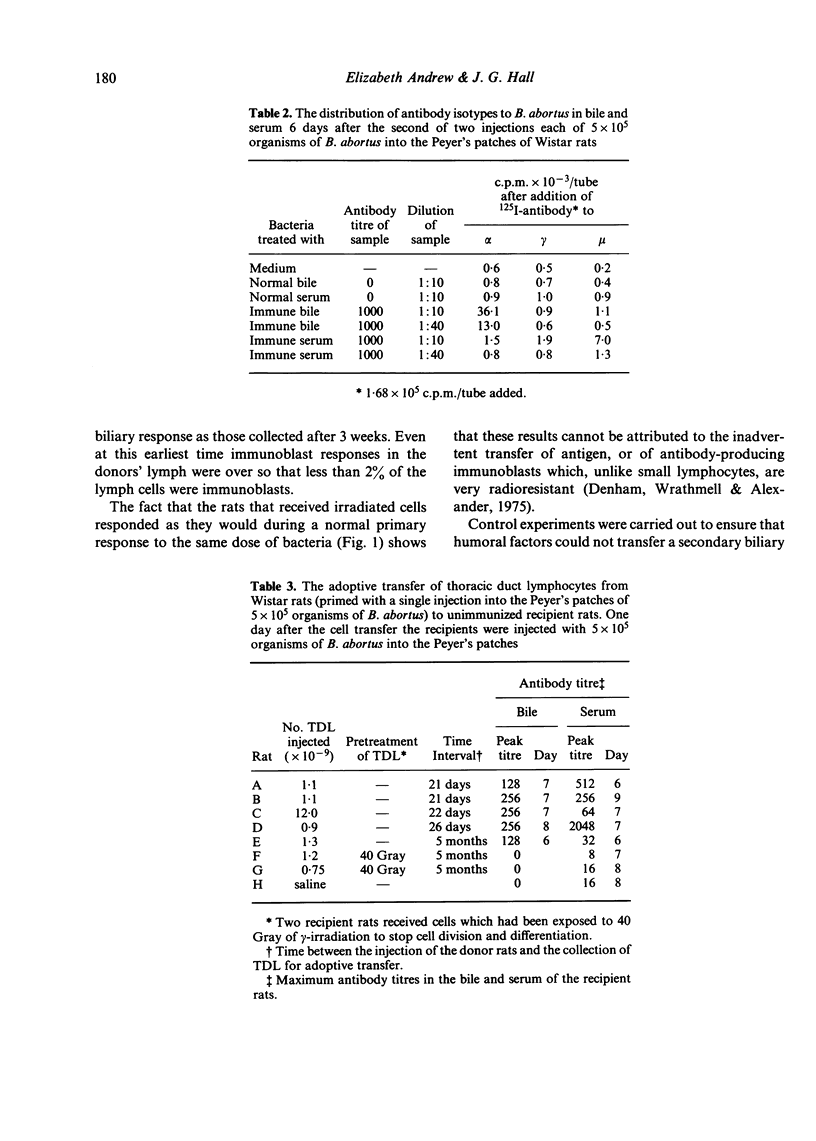
The Peyer's patches of Wistar rats were injected with suspensions of either sheep red blood cells (SRBC) or killed Brucella abortus organisms in doses that were insufficient to induce the appearance of biliary antibodies. The rats were challenged after periods ranging from 1 week to 1 year with the same dose of antigen given by the same route, and their bile was monitored for the appearance of specific antibodies. The test animals produced biliary antibodies to a much higher titre, and usually more rapidly, than control rats which had received the total dose of antigen as a single injection. As in primary responses, the biliary antibodies produced by challenging the primed rats were predominantly from the IgA class. The ability to mount substantial biliary responses to suboptimal doses of antigen could be transferred from primed donor rats to unimmunized recipients by thoracic duct lymphocytes, but not humoral factors, collected between 3 weeks and 5 months after priming. Gamma-irradiation of the lymphocytes abolished this effect. These results suggest strongly that immunological memory exists in the IgA system and that it is mediated by circulating lymphocytes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- André C., André F., Druguet M., Fargier M. C. Response of anamnestic IgA-producing cells in the mouse gut after repeated intragastric immunization. Adv Exp Med Biol. 1978;107:583–591. doi: 10.1007/978-1-4684-3369-2_66. [DOI] [PubMed] [Google Scholar]
- André C., Bazin H., Heremans J. F. Influence of repeated administration of antigen by the oral route on specific antibody-producing cells in the mouse spleen. Digestion. 1973;9(2):166–175. doi: 10.1159/000197442. [DOI] [PubMed] [Google Scholar]
- Denham S., Wrathmell A. B., Alexander P. Evidence of cytotoxic T and B immunoblasts in the thoracic duct of rats bearing tumour grafts. Transplantation. 1975 Feb;19(2):102–114. doi: 10.1097/00007890-197502000-00002. [DOI] [PubMed] [Google Scholar]
- Elson C. O., Heck J. A., Strober W. T-cell regulation of murine IgA synthesis. J Exp Med. 1979 Mar 1;149(3):632–643. doi: 10.1084/jem.149.3.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrman J. A., Cebra J. J. Special features of the priming process for a secretory IgA response. B cell priming with cholera toxin. J Exp Med. 1981 Mar 1;153(3):534–544. doi: 10.1084/jem.153.3.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowans J. L., Uhr J. W. The carriage of immunological memory by small lymphocytes in the rat. J Exp Med. 1966 Nov 1;124(5):1017–1030. doi: 10.1084/jem.124.5.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J., Orlans E., Reynolds J., Dean C., Peppard J., Gyure L., Hobbs S. Occurrence of specific antibodies of the IgA class in the bile of rats. Int Arch Allergy Appl Immunol. 1979;59(1):75–84. doi: 10.1159/000232242. [DOI] [PubMed] [Google Scholar]
- Husband A. J., Gowans J. L. The origin and antigen-dependent distribution of IgA-containing cells in the intestine. J Exp Med. 1978 Nov 1;148(5):1146–1160. doi: 10.1084/jem.148.5.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaitre-Coelho I., Jackson G. D., Vaerman J. P. Relevance of biliary IgA antibodies in rat intestinal immunity. Scand J Immunol. 1978;8(5):459–463. doi: 10.1111/j.1365-3083.1978.tb00542.x. [DOI] [PubMed] [Google Scholar]
- Mason D. W. The class of surface immunoglobulin on cells carrying IgG memory in rat thoracic duct lymph: the size of the subpopulation mediating IgG memory. J Exp Med. 1976 May 1;143(5):1122–1130. doi: 10.1084/jem.143.5.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mond J. J., Caporale L. H., Thorbecke G. J. Kinetics of B cell memory development during a thymus "independent" immune response. Cell Immunol. 1974 Jan;10(1):105–116. doi: 10.1016/0008-8749(74)90155-5. [DOI] [PubMed] [Google Scholar]
- Ogra P. L., Karzon D. T. Distribution of poliovirus antibody in serum, nasopharynx and alimentary tract following segmental immunization of lower alimentary tract with poliovaccine. J Immunol. 1969 Jun;102(6):1423–1430. [PubMed] [Google Scholar]
- Pierce N. F., Gowans J. L. Cellular kinetics of the intestinal immune response to cholera toxoid in rats. J Exp Med. 1975 Dec 1;142(6):1550–1563. doi: 10.1084/jem.142.6.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce N. F., Sack R. B., Sircar B. K. Immunity to experimental cholera. III. Enhanced duration of protection after sequential parenteral-oral administration of toxoid to dogs. J Infect Dis. 1977 Jun;135(6):888–896. doi: 10.1093/infdis/135.6.888. [DOI] [PubMed] [Google Scholar]
- Pierce N. F. The role of antigen form and function in the primary and secondary intestinal immune responses to cholera toxin and toxoid in rats. J Exp Med. 1978 Jul 1;148(1):195–206. doi: 10.1084/jem.148.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter P., Kenworthy R., Noakes D. E., Allen W. D. Intestinal antibody secretion in the young pig in response to oral immunization with Escherichia coli. Immunology. 1974 Nov;27(5):841–853. [PMC free article] [PubMed] [Google Scholar]


