Abstract
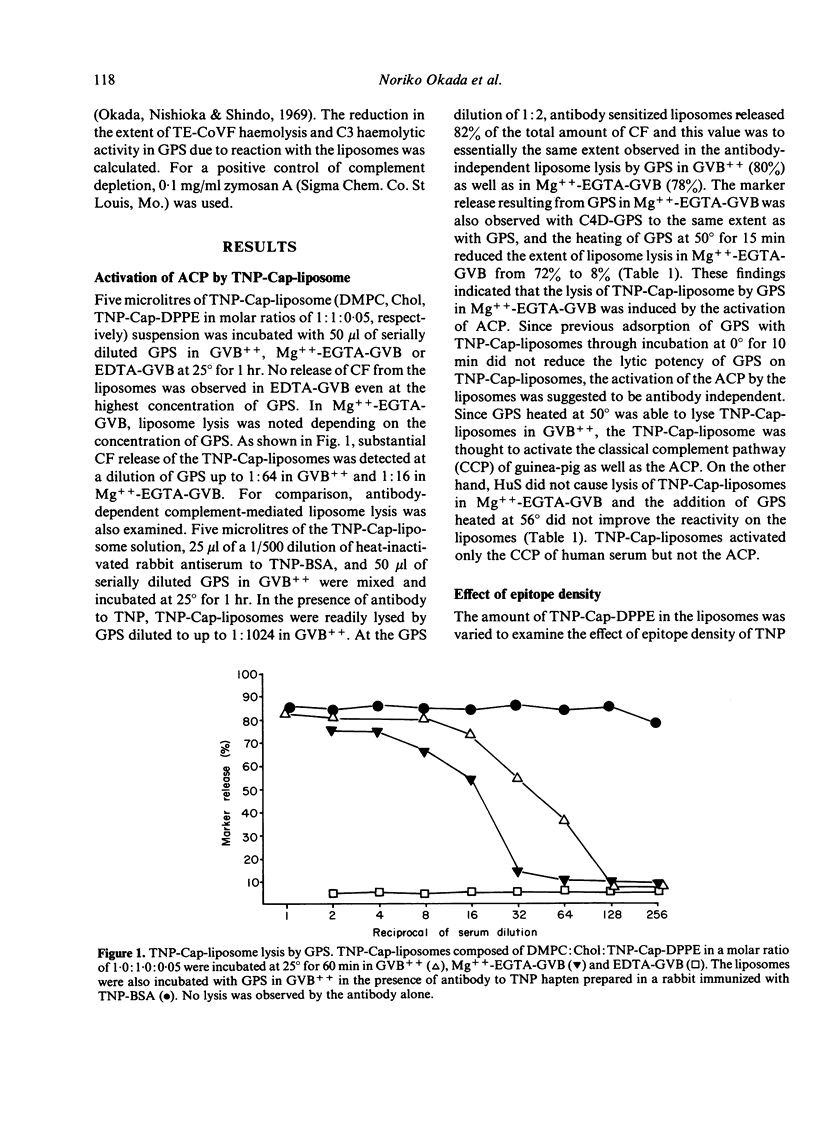
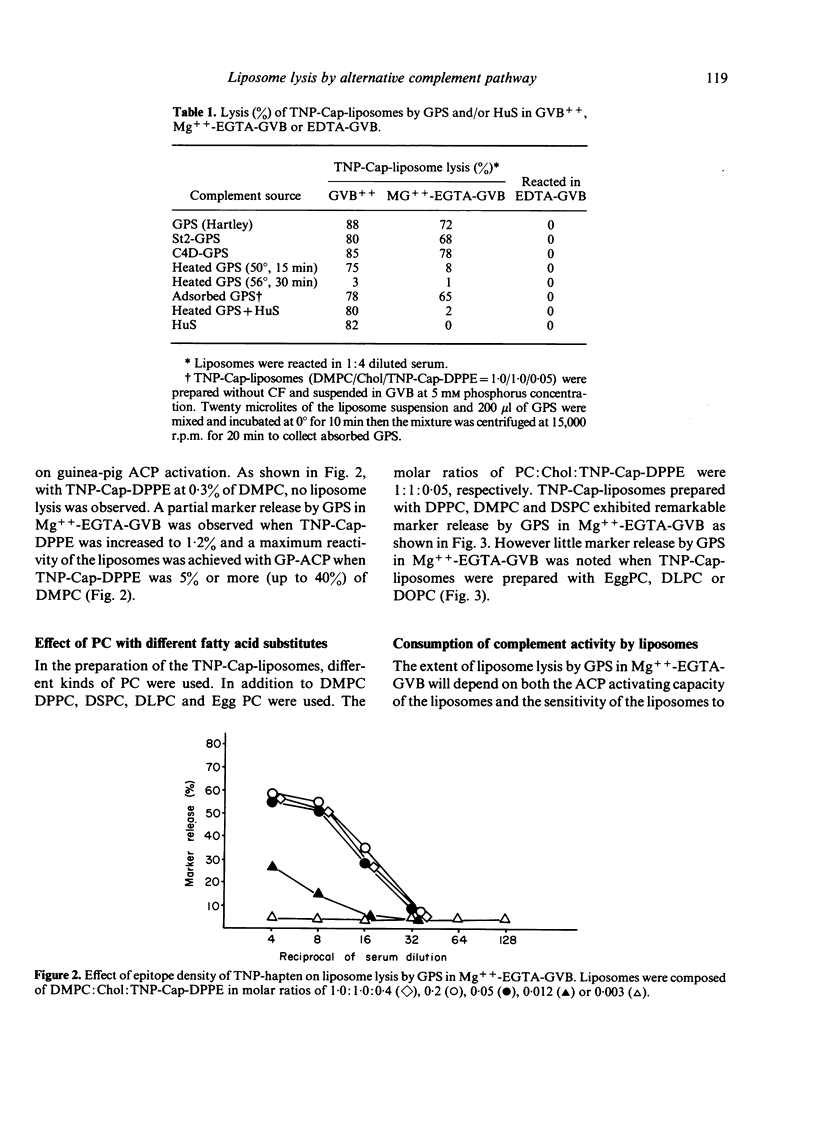
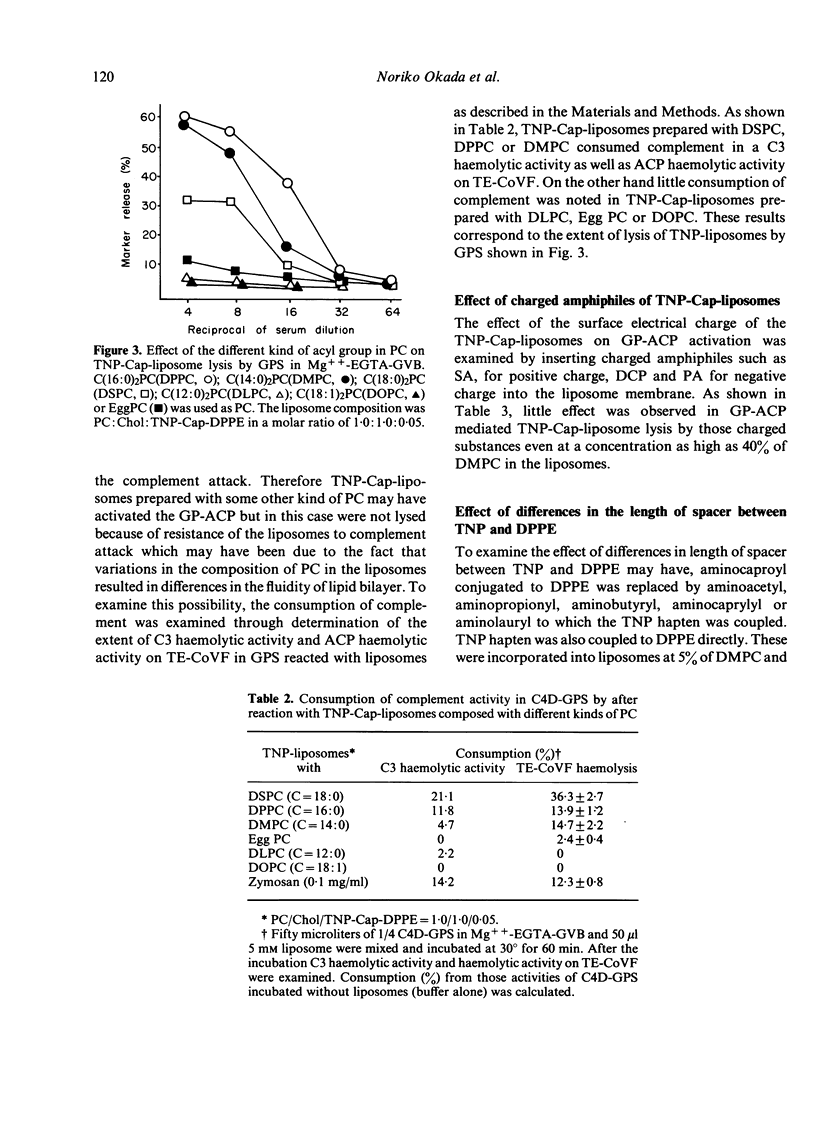
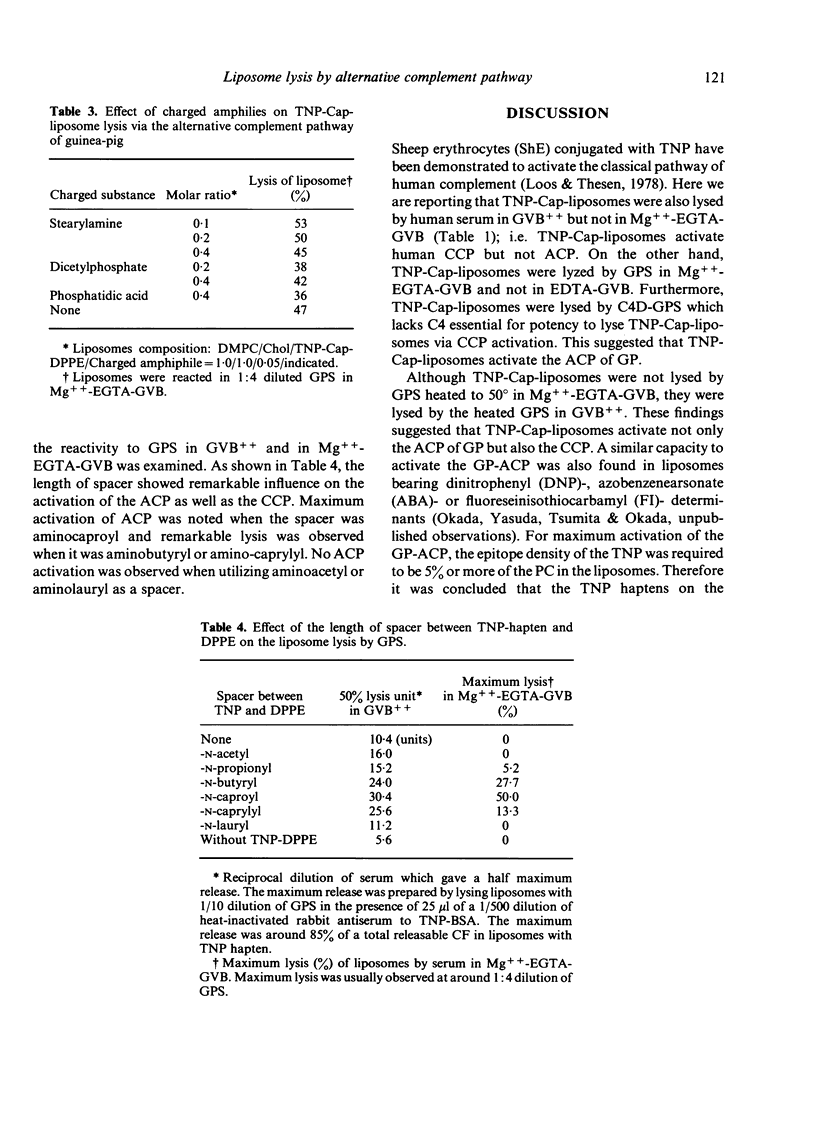
By incorporation of trinitrophenylamino-caproyldipalmitoylphosphatidylethanolamine (TNP-Cap-DPPE) into liposomes composed of an equimolecular mixture of dimyristoylphosphatidylcholine (DMPC) and cholesterol (TNP-Cap-liposomes), liposomes became readily lysed by guinea-pig serum (GPS) in Mg++-EGTA-GVB (gelatin veronal buffered saline containing 2 mM MgCl2 and 10 mM ethyleneglycol-bis (beta-amino-ethyl ether)N-N'-tetraacetate) as well as in GVB++ (gelatin veronal buffered saline containing 0.15 mM CaCl2 and 0.5 mM MgCl2). Since the classical complement pathway (CCP) does not work in Mg++-EGTA-GVB, TNP-Cap-liposome lysis by GPS in Mg++-EGTA-GVB was thought to be mediated by the activation of the alternative complement pathway (ACP). This conclusion was supported by observations that heating of GPS at 50 degrees impaired its lytic activity while C4-deficient GPS was capable of lytic activity, no lysis occurred in EDTA, and there was noted consumption of complement in GPS treated with TNP-Cap-liposomes at 30 degrees. For TNP-Cap-liposome lysis by GPS in Mg++-EGTA-GVB, the epitope density of the TNP hapten was required to be 5% or more of the DMPC. Changing the acyl group of the phosphatidylcholine (PC) significantly influenced the ACP activating capacity of TNP-Cap-liposome. Dipalmitoyl-PC, DMPC and distearoyl-PC facilitated the ACP activating capacity of the TNP-Cap-liposome, while dilauroyl-PC, egg-PC and dioleoyl-PC did not. Furthermore, the length of spacer between TNP and dipalmitoylphosphatidylethanolamine (DPPE) also influenced the ACP activating capacity and maximum activation was noted when the spacer was aminocaproyl. These physicochemical characteristics which increase the ACP activating capacity coincided with those reported to increase the immunogenicity of hapten-sensitized liposomes.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alper C. A., Rosen F. S., Lachmann P. J. Inactivator of the third component of complement as an inhibitor in the properdin pathway. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2910–2913. doi: 10.1073/pnas.69.10.2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger R., Hadding U., Schorlemmer H. U., Brade V., Bitter-Suermann D. Dextran sulphate: a synthetic activator of C3 via the alternative pathway. I. Influence of molecular size and degree of sulphation on the activation potency. Immunology. 1975 Sep;29(3):549–554. [PMC free article] [PubMed] [Google Scholar]
- Burger R., Hadding U., Schorlemmer H. U., Brade V., Bitter-Suermann D. Dextran sulphate: a synthetic activator of C3 via the alternative pathway. I. Influence of molecular size and degree of sulphation on the activation potency. Immunology. 1975 Sep;29(3):549–554. [PMC free article] [PubMed] [Google Scholar]
- Cunningham C. M., Kingzette M., Richards R. L., Alving C. R., Lint T. F., Gewurz H. Activation of human complement by liposomes: a model for membrane activation of the alternative pathway. J Immunol. 1979 Apr;122(4):1237–1242. [PubMed] [Google Scholar]
- Dancey G. F., Isakson P. C., Kinsky S. C. Immunogenicity of liposomal model membranes sensitized with dinitrophenylated phosphatidylethanolamine derivatives containing different length spacers. J Immunol. 1979 Feb;122(2):638–642. [PubMed] [Google Scholar]
- Fearon D. T., Austen K. F. Activation of the alternative complement pathway with rabbit erythrocytes by circumvention of the regulatory action of endogenous control proteins. J Exp Med. 1977 Jul 1;146(1):22–33. doi: 10.1084/jem.146.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T. Regulation by membrane sialic acid of beta1H-dependent decay-dissociation of amplification C3 convertase of the alternative complement pathway. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1971–1975. doi: 10.1073/pnas.75.4.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewurz H., Shin H. S., Mergenhagen S. E. Interactions of the complement system with endotoxic lipopolysaccharide: consumption of each of the six terminal complement components. J Exp Med. 1968 Nov 1;128(5):1049–1057. doi: 10.1084/jem.128.5.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa T., Inoue K. Effect of temperature on immune damage of liposomes prepared in the presence and absence of cholesterol. Nature. 1975 Mar 20;254(5497):254–256. doi: 10.1038/254254a0. [DOI] [PubMed] [Google Scholar]
- Loos M., Thesen R. Trinitrophenylated red cells (E-TNP) as a model for antibody-independent activation of the complement system via the classical pathway. J Immunol. 1978 Jul;121(1):24–28. [PubMed] [Google Scholar]
- Mold C., Gewurz H. Activation of human complement by liposomes: serum factor requirement for alternative pathway activation. J Immunol. 1980 Aug;125(2):696–700. [PubMed] [Google Scholar]
- Müller-Eberhard H. J. Complement. Annu Rev Biochem. 1975;44:697–724. doi: 10.1146/annurev.bi.44.070175.003405. [DOI] [PubMed] [Google Scholar]
- Nicol P. A., Lachmann P. J. The alternate pathway of complement activation. The role of C3 and its inactivator (KAF). Immunology. 1973 Feb;24(2):259–275. [PMC free article] [PubMed] [Google Scholar]
- Okada H., Baba T. Rosette formation of human erythrocytes on cultured cells of tumour origin and activation of complement by cell membrane. Nature. 1974 Apr 5;248(448):521–522. doi: 10.1038/248521a0. [DOI] [PubMed] [Google Scholar]
- Okada H., Kawachi S., Nishioka K. A new complement inhibitor in guinea pig serum. Jpn J Exp Med. 1969 Oct;39(5):527–531. [PubMed] [Google Scholar]
- Okada H., Nishioka K., Sindo T. The effects of Cu-chlorophyllin on the active site formation of each component of guinea-pig complement. Immunology. 1969 Apr;16(4):473–480. [PMC free article] [PubMed] [Google Scholar]
- PILLEMER L., SCHOENBERG M. D., BLUM L., WURZ L. Properdin system and immunity. II. Interaction of the properdin system with polysaccharides. Science. 1955 Sep 23;122(3169):545–549. doi: 10.1126/science.122.3169.545. [DOI] [PubMed] [Google Scholar]
- Pangburn M. K., Morrison D. C., Schreiber R. D., Müller-Eberhard H. J. Activation of the alternative complement pathway: recognition of surface structures on activators by bound C3b. J Immunol. 1980 Feb;124(2):977–982. [PubMed] [Google Scholar]
- Richards R. L., Gewurz H., Osmand A. P., Alving C. R. Interactions of C-reactive protein and complement with liposomes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5672–5676. doi: 10.1073/pnas.74.12.5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruddy S., Austen K. F. C3b inactivator of man. II. Fragments produced by C3b inactivator cleavage of cell-bound or fluid phase C3b. J Immunol. 1971 Sep;107(3):742–750. [PubMed] [Google Scholar]
- Schenkein H. A., Ruddy S. The role of immunoglobulins in alternative complement pathway activation by zymosan. I. Human IgG with specificity for Zymosan enhances alternative pathway activation by zymosan. J Immunol. 1981 Jan;126(1):7–10. [PubMed] [Google Scholar]
- Six H. R., Uemura K. I., Kinsky S. C. Effect of immunoglobulin class and affinity on the initiation of complement-dependent damage to liposomal model membranes sensitized with dinitrophenylated phospholipids. Biochemistry. 1973 Sep 25;12(20):4003–4011. doi: 10.1021/bi00744a034. [DOI] [PubMed] [Google Scholar]
- Tadakuma T., Yasuda T., Kinsky S. C., Pierce C. W. The effect of epitope density on the in vitro immunogenicity of hapten-sensitized liposomal model membranes. J Immunol. 1980 May;124(5):2175–2179. [PubMed] [Google Scholar]
- Tamura N., Nelson R. A., Jr Three naturally-occurring inhibitors of components of complement in guinea pig and rabbit serum. J Immunol. 1967 Sep;99(3):582–589. [PubMed] [Google Scholar]
- Uemura K., Kinsky S. C. Active vs. passive sensitization of liposomes toward antibody and complement by dinitrophenylated derivatives of phosphatidylethanolamine. Biochemistry. 1972 Oct 24;11(22):4085–4094. doi: 10.1021/bi00772a010. [DOI] [PubMed] [Google Scholar]
- Weiler J. M., Daha M. R., Austen K. F., Fearon D. T. Control of the amplification convertase of complement by the plasma protein beta1H. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3268–3272. doi: 10.1073/pnas.73.9.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whaley K., Ruddy S. Modulation of the alternative complement pathways by beta 1 H globulin. J Exp Med. 1976 Nov 2;144(5):1147–1163. doi: 10.1084/jem.144.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda T., Naito Y., Tsumita T., Tadakuma T. A simple method to measure anti-glycolipid antibody by using complement-mediated immune lysis of fluorescent dye-trapped liposomes. J Immunol Methods. 1981;44(2):153–158. doi: 10.1016/0022-1759(81)90342-2. [DOI] [PubMed] [Google Scholar]


