Abstract
A comparative study has been made of human precipitating and co-precipitating anti-tetanus toxin antibodies. IgG co-precipitating antibody represented 10% of the total antibodies in the serum and had immunological and biological properties similar to those described for co-precipitating antibodies of other animal species.
Human precipitating and co-precipitating antibodies had the same electrophoretic mobility and were localized in the same immunoglobulin fraction. By immunoprecipitation it was not possible to find antigenic differences between precipitating and co-precipitating antibodies. Both antibodies were localized in the IgG1 and IgG3 subclasses and neither were in the IgG4 subclass. Only the precipitating antibody can form insoluble complexes with antigen. Precipitating and co-precipitating antibodies agglutinated sensitized sheep red cells, however, only the precipitating antibody agglutinated human red cells. Eight to ten times more co-precipitating antibody was required to obtain a positive reaction in PCA.
Precipitating antibody activated the complement system while co-precipitating antibody lacked this capacity. This difference in behaviour could not be attributed to localization of both antibodies in different IgG subclasses.
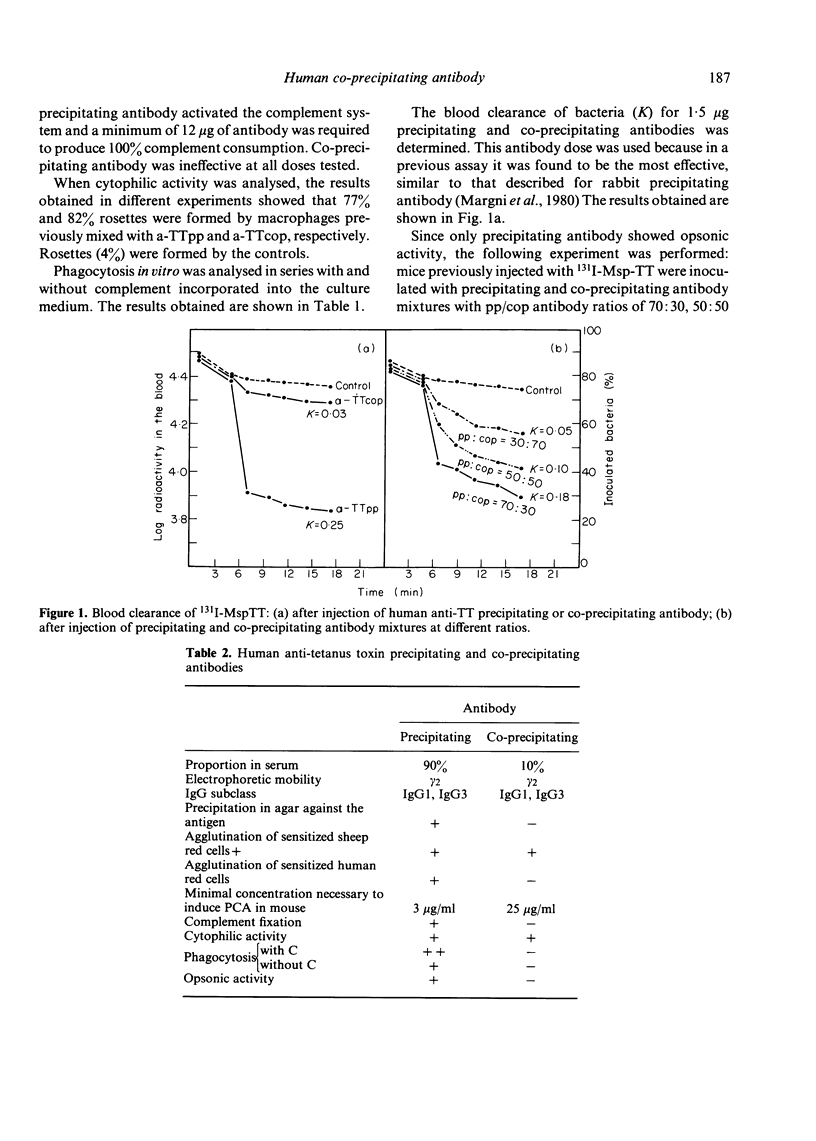
Precipitating and co-precipitating antibodies were cytophilic. Only the former activated phagocytosis and increased clearance of antigen from the blood. These results are not surprising since co-precipitating antibody does not fix complement. Competition between human precipitating and co-precipitating antibodies in opsonization was analysed. In this test competition of both antibodies for the antigen depends on their respective amounts. The K = 0.18 diminished to 0.05 when the ratio of pp:cop. antibody changed from 70:30 to 30:70.
The fact that co-precipitating antibody was isolated from the sera of vertebrates other than man indicate that this antibody could possibly play a role in some immune mechanisms. Taking into account that in previous papers we have demonstrated that co-precipitating antibody functions as a molecule with one combining site of high affinity and one of low affinity, we have proposed that this antibody could function univalently and blocks the antigen. This could facilitate chronic parasitic, bacterial and viral infections, tumour growth and other chronic infections.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cordal M. E., Margni R. A. Isolation, purification and biological properties of horse precipitating and non precipitating antibodies. Immunochemistry. 1974 Dec;11(12):765–770. doi: 10.1016/0019-2791(74)90295-x. [DOI] [PubMed] [Google Scholar]
- Del Guercio P., Tolone G., Andrade F. B., Biozzi G., Binaghi R. A. Opsonic, cytophilic and agglutinating activity of guinea-pig gamma-2 and gamma-M anti-Salmonella antibodies. Immunology. 1969 Mar;16(3):361–371. [PMC free article] [PubMed] [Google Scholar]
- Forget A., Borduas A. G. An immunological enhancement phenomenon in experimental brucella infection of the chicks. Int Arch Allergy Appl Immunol. 1977;53(2):190–194. doi: 10.1159/000231751. [DOI] [PubMed] [Google Scholar]
- Hajos S. E., Margni R. A., Perdigón G., Manghi M., Olivera R. Binding of immunoglobulins and immune complexes to erythrocytes of vertebrates. Immunochemistry. 1978 Sep;15(9):623–628. doi: 10.1016/0161-5890(78)90034-2. [DOI] [PubMed] [Google Scholar]
- LATHAM W. C., BENT D. F., LEVINE L. Tetanus toxin production in the absence of protein. Appl Microbiol. 1962 Mar;10:146–152. doi: 10.1128/am.10.2.146-152.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margni R. A., Acerbo E. O., Heer E., Hajos S. E. The importance of immunochemical analysis in the differential diagnosis of microglobulinic myelomas. Clin Chim Acta. 1970 Jun;28(3):451–456. doi: 10.1016/0009-8981(70)90072-0. [DOI] [PubMed] [Google Scholar]
- Margni R. A., Cordal M. E., Leoni J., Hajos S. E., Veira S., Manghi M., Bazzurro M. Non-precipitating antibodies isolated by immunoadsorption. Immunochemistry. 1977 Apr;14(4):299–303. doi: 10.1016/0019-2791(77)90253-1. [DOI] [PubMed] [Google Scholar]
- Margni R. A., Hajos S. E., Cordal M. E., Quiroga S. The haemagglutinating activity of different anti-DNP antibody populations when dinitrophenylated sheep and human red cells are used as agglutinogen. J Immunol Methods. 1976;13(1):51–62. doi: 10.1016/0022-1759(76)90186-1. [DOI] [PubMed] [Google Scholar]
- Margni R. A., Hajos S. E., Perdigón G., Manghi M., Borel I. M. Ontogenic evolution of chicken red cell Fc receptor. Cell Immunol. 1979 Nov;48(1):235–237. doi: 10.1016/0008-8749(79)90116-3. [DOI] [PubMed] [Google Scholar]
- Margni R. A., Hajos S. Biological and physicochemical properties of purified anti-DNP guinea-pig non-precipitating antibodies. Immunology. 1973 Mar;24(3):435–443. [PMC free article] [PubMed] [Google Scholar]
- Margni R. A., Hajos S., Manghi M., Perdigón G., Leoni J. Red cell Fc receptors and their participation in the passive haemagglutination mediated by non-precipitating antibodies. Immunology. 1974 Nov;27(5):863–870. [PMC free article] [PubMed] [Google Scholar]
- Margni R. A., Paz C. B., Cordal M. E. Immunochemical behavior of sheep non-precipitating antibodies isolated by immunoadsorption. Immunochemistry. 1976 Mar;13(3):209–214. doi: 10.1016/0019-2791(76)90217-2. [DOI] [PubMed] [Google Scholar]
- Margni R. A., Perdigón G., Abatángelo C., Gentile T., Binaghi R. A. Immunobiological behaviour of rabbit precipitating and non-precipitating (co-precipitating) antibodies. Immunology. 1980 Nov;41(3):681–686. [PMC free article] [PubMed] [Google Scholar]
- Margni R., Binaghi R. Purification and properties of non-precipitating rabbit antibodies. Immunology. 1972 Apr;22(4):557–563. [PMC free article] [PubMed] [Google Scholar]
- McCutchan J. A., Katzenstein D., Norquist D., Chikami G., Wunderlich A., Braude A. I. Role of blocking antibody in disseminated gonococcal infection. J Immunol. 1978 Nov;121(5):1884–1888. [PubMed] [Google Scholar]
- OVARY Z. [Cutaneous anaphylaxis in the albino rat]. Int Arch Allergy Appl Immunol. 1952;3(4):293–301. doi: 10.1159/000227977. [DOI] [PubMed] [Google Scholar]
- SCHEIDEGGER J. J. Une micro-méthode de l'immuno-electrophorèse. Int Arch Allergy Appl Immunol. 1955;7(2):103–110. [PubMed] [Google Scholar]


