Abstract
Objective:
To evaluate the neuromuscular activation profiles of trunk muscles in commonly used gymnastic strength exercises with a polymyographic set-up and to describe the training effects of each exercise.
Design and Setting:
Subjects performed 9 repetitions of each of 12 gymnastic exercises. Variations of 5 trunk flexions, 5 extensions, and 2 lateral-flexion movements were performed under standardized test conditions.
Subjects:
Ten healthy subjects (men and women) who were familiar with the exercises participated in the study.
Measurements:
We recorded surface electromyograms (EMGs) from the rectus abdominis, external oblique, rectus femoris, middle trapezius, erector spinae at T12 and L3, gluteus maximus, and semitendinosus and semimembranosus muscles. Recording of each repetition cycle was triggered by a flexible electronic goniometer attached to the trunk. The raw EMG signals were rectified, smoothed, amplitude normalized to maximal voluntary contraction (MVC), and averaged for the last 8 repetitions.
Results:
Pure spine-flexion exercises, such as a curl-up, produced sufficient and isolated activation (greater than 50% MVC) of the abdominal muscles. When flexion of the spine was combined with hip flexion (sit-up), the peak activation was increased. Lateral-flexion tasks targeted primarily the external oblique muscle, which demonstrated high activity in side-lying flexion tasks. Back- and hip-extension exercises, such as bridging and diagonal hip and shoulder extension, produced only moderate mean activities (less than 35% MVC) in the trunk-extensor muscles. Trunk-extension exercises with combined hip extension increased the EMG activity to 50% MVC but only at the end of the extension.
Conclusions:
Individual responses to each exercise varied markedly, which complicated the classification of exercise effects. However, within the limitations of the study, we found that the chosen abdominal exercises provided an effective training stimulus for the trunk-flexor muscles, whereas in the back- and hip-extension exercises, the neuromuscular activation tended to be too low or unspecific to qualify as muscle-specific training.
Keywords: electromyography, activation profiles, EMG normalization, EMG variability, movement standardization, back muscles, abdominal muscles, hip muscles, training effectiveness
A wide variety of different trunk exercises are currently used for training and conditioning purposes, both in athletic programs (eg, competitive sports and fitness) and in rehabilitation practice (eg, low back pain patients and back schools). The effectiveness of neuromuscular training is typically based on functional anatomical evaluations, empiric measurements, or subjective perception. Despite the large number of different exercise set-ups, scientific evaluation of their specific effects on the targeted musculature is lacking. The fundamental questions are (1) is the muscle active and (2) if yes, is its activation high enough and long enough to elicit a training response for strength or endurance improvement?
Surface electromyography (EMG) can be used as a quantitative method to detect the activation level and patterns of muscle groups in movement.1 A review of the existing EMG literature related to this topic indicates that many study findings are limited by poor standardization, insufficient EMG processing, or missing statistical analyses. Typically, a qualitative approach of EMG calculation based on microvolts, ordinal scaling (more or less activity), or both was used.2–4
Recent investigators5–10 have used state-of-the-art EMG methods incorporating fine-wire electrodes, amplitude-normalization techniques, and interfacing with other biomechanical sensors to evaluate the neuromuscular function of trunk and hip muscles in a wide variety of daily activities and training exercises. One drawback of most studies examining back and hip extension is the lack of control or detection of the hip-extensor muscles, such as the gluteus maximus and the hamstring muscles. We found no study detecting the dorsal and ventral “chain” of the main trunk and hip muscles within one measurement set-up.
The purpose of our study was to record both the dorsal and ventral superficial muscles simultaneously to demonstrate the activation and coactivation patterns of the main trunk and hip muscles. The EMG activation profiles for the main movements of the spine (extension, flexion, lateral flexion, and rotation) were determined in gymnastic exercises. Standardized methods and quantitative EMG analysis incorporating the latest amplifier technology were used to allow for comparisons among the exercises.
METHODS
We investigated 10 healthy subjects (3 women, 7 men; age, 27.8 ± 2.4 years; body weight, 75.8 ± 15.8 kg; height, 177.9 ± 10.4 cm). All subjects were familiar with strength training and gymnastic exercises, but none were specifically training at that time. Informed consent was obtained from each subject before participation in the study. Because the study was conducted at a sports institution rather than a medical facility, institutional review board approval was not required.
Trunk-training exercises were performed in randomized order: 12 gymnastic exercises (Figure 1), including 5 for trunk and hip flexion, 2 for trunk lateral flexion, and 5 for trunk and hip extension. After a standardized sequence of warm-up on a bicycle ergometer and stretching exercises, each subject performed 12 different static maximal voluntary contractions (MVCs), and each contraction (of 3 to 5 seconds' duration) was repeated 2 times (Figure 2). The rationale for these exercises is based on comprehensive pilot studies of the most effective task to produce maximum EMG activity. All subjects were familiar with the MVC tasks, especially with the machine exercises, on which they had trained extensively in the past. After the MVC set, the subjects performed 9 repetitions for each training exercise. The contraction duration was standardized by using an acoustic metronome at 30 beeps per minute. Between sets was a rest period of at least 5 minutes.
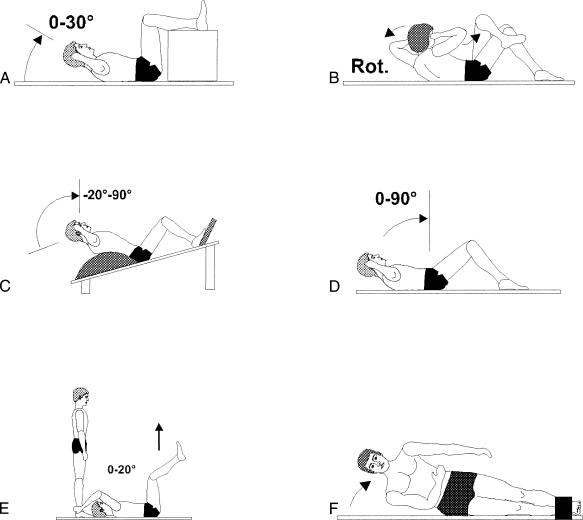
Figure 1.
Gymnastic training exercises. A, Straight Curl-Up. Fingertips touch the temples, arms are in a fixed lateral position, the head and shoulders are lifted, and the feet are not fixed. B, Cross Curl-Up. As in A, but 1 leg is across the other, and the contralateral elbow is moved to the opposite knee. C, Curl Up, Hyperextended. Same arm position as A but inverse (-20°) starting position; trunk and hip flexion until the head and thorax are upright; no foot fixation. D, Sit-Up. Same arm position as A; trunk and hip flexion until the upper body is upright; no foot fixation. E, Vertical Hip Lift. Knees are flexed between 70° and 90°, arms are fixed, hip is lifted until lumbar spine is lifted from the ground. F, Lateral Flexion, Fixed Legs. Foot of upper leg is crossed over the lower leg and fixed; flexion until the upper body is lifted off the ground (30°). G, Lateral Hip Lift. Elbow support from a flexed position (30° from hip to ground), extension to the neutral position (0°). H, Diagonal Hip and Shoulder Extension. From a flexed position (elbow in contact with the contralateral knee), diagonal hip and shoulder extension to the horizontal position. I, Kneeling Back Extension. Same arm position as A, from a flexed position (chest-leg contact), isolated spine extension (head and thorax to 45°). J, Trunk Extension, Fixed Legs. Same arm position as A, fixed legs in prone position, from 90° hip flexion-extension to the horizontal (0°). K, Bridging. Supine position, trunk and arms resting on ground and knees bent (90°), hip extension to neutral position (0°). L, Hip Extension, Fixed Trunk. Fixed upper body in prone position, from 90° hip and knee flexion with extension of legs to the horizontal line (hip and knee, 0°).
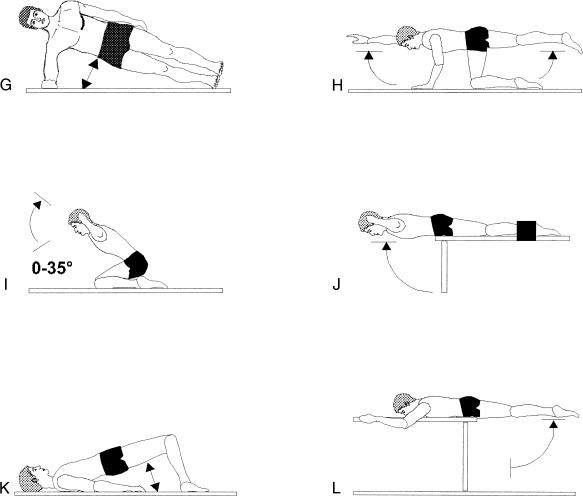
Figure 2.
Static test positions used to determine the maximum voluntary contraction. Unless otherwise indicated (by arrow, straps, or lever arm), the static resistance was provided manually by assistants. The numbers below each exercise indicate, separately for each muscle, the number of subjects who reached their highest activity level with this exercise.
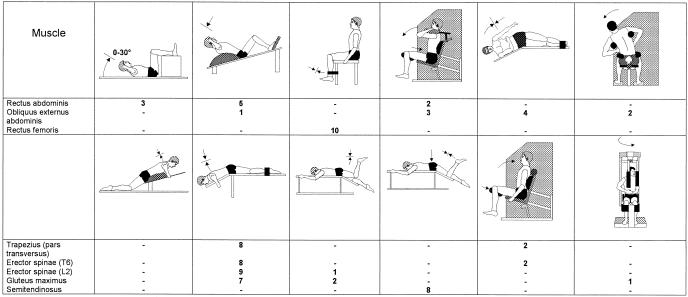
We recorded surface EMG signals from 8 muscles; the dorsal muscle extension chain was represented by 5 muscle groups and the ventral muscle chain by 3 muscle groups (Table 1).
Table 1.
Muscles and Electrode Positions for the Surface Electromyogram Measurements
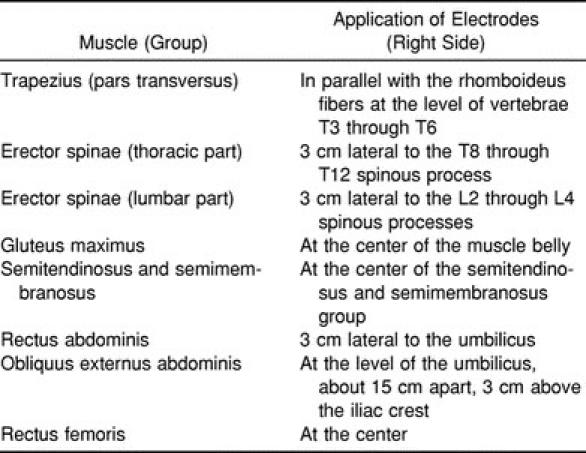
Wet-gel, nondisposable, 1.2-cm electrodes (Type Blue Sensor P00S, Medicotest, Ølstykke, Denmark) were applied parallel to the muscle-fiber orientation, with an interelectrode distance of 2 cm. We prepared the skin by using a special abrasive-conductive fluid that rubs and cleans the skin (Every, Neurodata, Vienna, Austria). Care was taken that interelectrode impedance was less than 10 kOhm. We tested the signal quality by visual inspection of the baseline while moving the cable and shaking the muscle. We performed spectral analysis on selected signals and analyzed the power spectrum to inspect the EMG quality and detect possible noise interference at 50 Hz.
The raw EMG data were measured at a bandwidth of 10 to 500 Hz, using a differential amplifier (MyoSystem 2008, Noraxon Inc, Scottsdale, AZ). According to the manufacturer's technical specifications, the common mode rejection ratio was greater than 110 dB, amplifier noise was less than 7 μVrms, and input impedance was equal to 10 mOhm. The signals were A/D converted with 1500 Hz and stored in a personal computer. The stored data first were full-wave rectified, then smoothed with a root mean square (150 milliseconds), and finally amplitude normalized to the highest activity level found in the set of MVC contractions (mean amplitude for 1 second). To define the start and end of each flexion-extension cycle for gymnastic exercises, a 2-dimensional goniometer (Penny & Giles Computer Products Ltd, Christchurch-Dorset, United Kingdom) was applied to the lateral trunk axis of the subjects. Because of possible starting effects, the first repetition of each set was excluded from the analysis; the remaining 8 repetitions were time normalized to 100 data points and expressed as an averaged repetition cycle ranging from 0% to 100%.11 Finally, for each exercise, these ensemble averages were averaged again for all subjects included in the study.
The coefficient of variation (CV) was used to describe the variability of EMG data,11 and the Pearson correlation coefficient was used to describe the similarity of EMG activation patterns. We used an analysis of variance for repeated measures to show the effect of different exercise tasks on the EMG amplitude within a muscle group and the post hoc multiple-comparison Newman-Keuls test to identify significant differences (P < .05) in mean values between tests.
RESULTS
Variability in Movement Execution
To standardize the tests, we asked all subjects to move with a cadence of 30 beeps per minute. The mean contraction duration for the first movement period (flexion, lateral flexion to the right side) of all 12 exercises was 1928 milliseconds, and the mean duration of the second movement period (extension, lateral flexion to the left) was 2031 milliseconds. A general trend indicated that the period to overcome load (concentric period) was performed faster than the backward movement (eccentric period). The mean duration difference between the periods was 254 milliseconds. The CV was calculated for each exercise to describe the variability of the contraction duration. For the concentric part of the movement, the mean CV for all exercises was 4.42%; for the eccentric period, 3.7%. The vertical and the lateral hip lift demonstrated values higher than 10% (maximum, 12.3%). The constancy of the range of motion (ROM) was calculated from the CV of the mean angle values obtained during each exercise. The average CV for the ROM was 10.68%.
Mean Activity Distribution Profiles
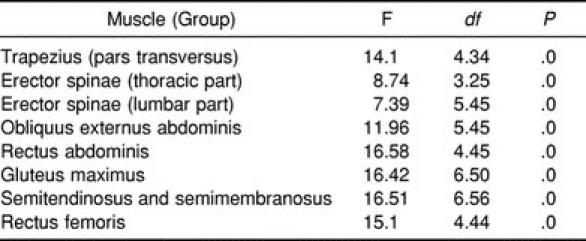
The analysis of variance revealed a significant effect for the exercise tasks for all muscles (Table 2). However, due to the EMG-specific variability (see the following sections), many differences among given tasks could not be confirmed by the post hoc multiple-comparison analysis. If not otherwise indicated in the text, the findings were not significant.
Table 2.
Univariate Analysis of Variance of Effect of Exercise Variation on Muscle Activity
Gymnastic Abdominal-Flexion Exercises
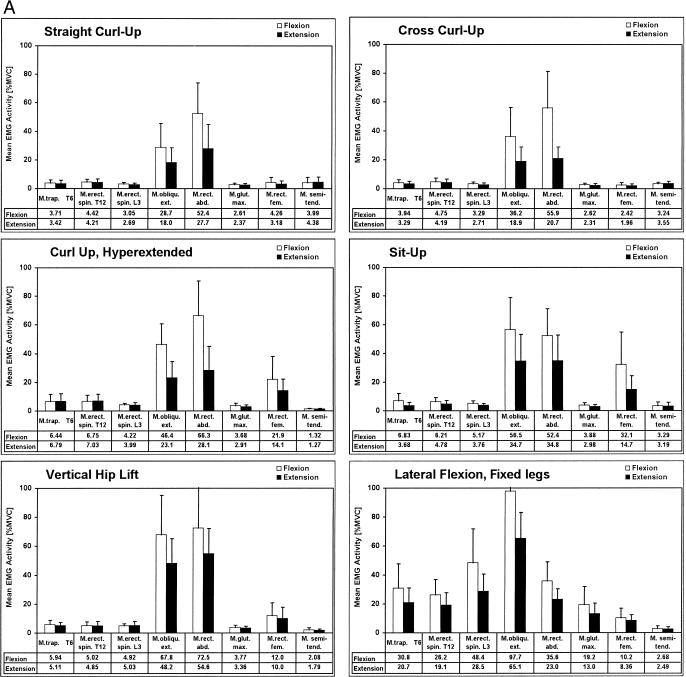
To investigate which muscle is involved in each exercise and what its activation level is for a given task, we calculated the mean EMG activity for each period within the averaged repetition cycle (Figure 3). Three exercises, the straight curl-up, the cross curl-up, and the vertical hip lift, were spine-flexion tasks without combined hip flexion. The distribution of EMG activity over the ventral muscles indicated remarkable isolation of the abdominal muscles. In the straight curl-up, rectus abdominis activity showed a middle exhaustion level (52.45% MVC) for the flexion period, which was slightly but not significantly increased when additional rotation was added in the cross curl-up exercise (55.96% MVC). External oblique muscle activity was increased from 28.71% to 36.2% MVC when the straight curl-up was varied to the cross curl-up. The next 2 exercises, the curl-up, hyperextended at prestretched start position, and the regular sit-up, added hip flexion to the spine flexion. For both abdominal muscles, flexion activity increased when the straight curl-up was changed to the curl-up, hyperextended; the increase of 17.78% MVC for the oblique muscle was significant, but the increase of 13.87% MVC for the rectus abdominis muscle was not significant. When the straight curl-up was varied to a sit-up, the flexion activity level of the rectus abdominis muscle was unchanged, but the oblique muscle showed a significant increase from 28.71% to 56.51% MVC. Activity of the rectus femoris muscle, the only hip-flexor muscle evaluated in this study, increased significantly from less than 5% MVC (curl-up exercises) to 21.98% (curl-up, hyperextended) and 32.18% MVC (sit-up), indicating that the hip flexors were also activated in these exercises.
Figure 3.
Mean activity distribution profiles. The mean electromyogram activity for the concentric and eccentric periods is calculated as the mean of 8 repetitions, which is averaged again for all subjects (n = 10). The thin lines indicate 1 SD. A, Profiles for exercises 1 through 6. B, Profiles for exercises 7 through 12.
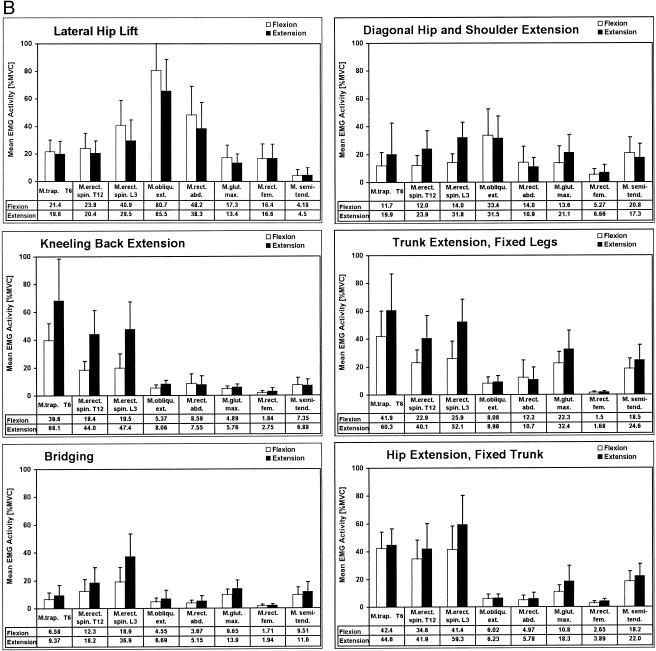
The most demanding exercise was the vertical hip lift, in which activation of all ventral muscles increased significantly. The coactivation of the dorsal extensor muscles in the flexion period of flexion tasks typically ranged from 2% to 7% MVC. The neuromuscular activation profiles of the 2 lateral flexion exercises illustrated their characterization as “whole-body” exercises: all trunk muscles showed considerable EMG activity. Most dominant was the flexion activity of the external oblique muscle in the lateral-flexion, fixed-legs exercise: almost-maximal EMG levels were reached (97.77% MVC). This high level was reduced in the lateral hip lift (80.78% MVC), in which lateral flexion was performed as hip flexion with foot and elbow support.
Gymnastic Back- and Hip-Extension Exercises
The mean muscle activity for the diagonal hip- and shoulder-extension exercise did not exceed 35% MVC for any of the muscles, despite the fact that all muscles were active. The kneeling back-extension exercise showed a remarkable isolation effect for the erector spinae muscles, which demonstrated extension activity levels of 68.12% MVC (trapezius muscle at T6) and 44.02% MVC (erector spinae muscle at T12). Compared with the trunk extension, fixed-legs exercise, in which hip extension is added to the spine extension, only the lumbar erector spinae extension activity was slightly increased by 4.77%. The hip extensors had comparatively lower mean activation of 32.44% MVC (gluteus maximus) and 24.6% MVC (semitendinosus and semimembranosus muscle) in the extension phase. In the bridging exercise, all dorsal extensor muscles were activated on a low level: the highest mean extension activity for this exercise was only 36.96% MVC for the lumbar erector spinae.
In the hip extension, fixed-trunk exercise, the lower body moves against the fixed upper body, opposite to the trunk extension with fixed legs. When these 2 exercises were compared, a similar activity distribution was found. However, the lumbar erector spinae EMG activation for extension was increased by 7.12% MVC, and trapezius and rhomboideus activity at level T6 was diminished by 15.5% MVC.
Averaged Activation Profiles
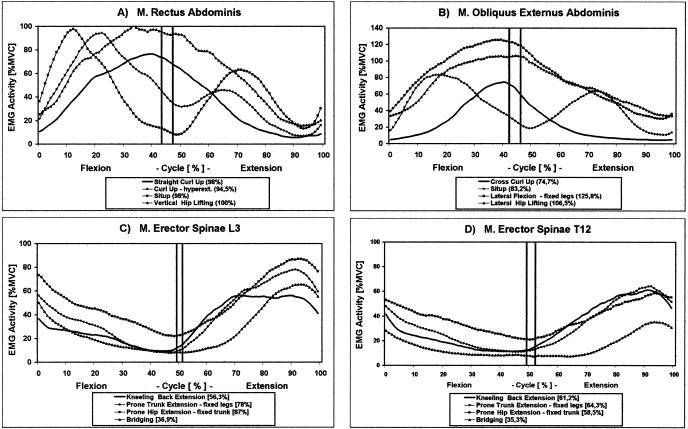
To demonstrate the development of EMG activity within a movement cycle, we analyzed the time- and amplitude-normalized activation profiles for selected target muscles (Figure 4). Clear differences in the cycle-specific activation can be seen for both abdominal muscles: the rectus abdominis had a single peak pattern in flexion exercises (eg, straight curl-up and vertical hip lift) and a biphasic pattern in combined spine- and hip-flexion movements (eg, curl-up, hyperextended, and sit-up). High activation peaks between 80% and 100% MVC were found at the beginning (curl-up, hyperextended, and sit-up) or at the end (straight curl-up, vertical hip lift) at the end of the flexion period.
Figure 4.
A through D, Time and maximal voluntary contraction–normalized electromyogram activation profiles (n = 10) of 4 target muscles in selected trunk exercises. The curves are based on individual average curves of 8 repetitions. The vertical lines indicate the range for point of return in these exercises. The peak activation value of each curve is specified in parentheses after each curve name. The corresponding mean values for each period and exercise are listed in Figure 3
As a general trend, the external oblique muscle showed a similar activation profile for flexion movements as the rectus abdominis but on a different level. For lateral-flexion tasks, local supramaximal peak activations occurred at the end of the flexion period (Figure 4B). Similar EMG activation patterns of the 2 portions of the erector spinae were found in the back-extension exercises (Figure 4C and D): a descending activation within the flexion period was followed by a constant increase in the extension period, in which peak activity between 65% and 87% MVC (lumbar erector spinae) and 35% and 65% MVC (thoracic erector spinae) occurred at the end of the movement cycle. A somewhat different shape was found for the lumbar erector spinae in the kneeling back-extension exercise, in which a constant activation level between 50% and 60% MVC, rather than a peak through the middle range of the extension period, was found.
DISCUSSION
Variability of Tests
Movement velocity and acceleration, ROM, and load are important factors that directly alter EMG amplitude.12,13 One approach to standardizing the velocity of movement within a set of repetitions is to use a metronome to control the duration of contraction.5–6,8,14–17 When combined with a standardized ROM, we can assume that the mean velocity of movement is nearly constant. Yet this does not automatically mean that the shortening and lengthening velocity of the muscle fibers is constant throughout the ROM and that temporary differences in acceleration are eliminated. We instructed our subjects to perform a smooth and controlled movement to minimize the effects of fluctuation in velocity and acceleration. The mean CV for the contraction duration, both for the concentric and eccentric contraction periods, was less than 5%, which is an acceptable value. Godfrey et al2 investigated the effect of different movement velocities in sit-up exercises and observed higher amplitudes for faster movements. They suggested that the discrepancies in the literature were due to differences in the cadence of movements. In our study, movement velocity differed among exercises because of varied ROMs. In some exercises, such as the vertical hip lift or lateral flexion with fixed legs, the duration variability among subjects increased up to 12%, reflecting the difficulty of the motor task (complexity, load, or both). Interindividual differences in the combined hip joint and segmental spine movement could have been one reason for increased variability, even if the overall movement of the whole body had a standardized range. Because of the multisegmental character of spine and hip movements, there is remarkable individual freedom to solve the given motor task.18
EMG Variability and Normalization
Our subjects performed a set of static exercises according to the concept proposed by McGill et al8–10,15 to achieve a valid MVC reference value. This approach is based on their observation that subjects do not all perform at maximum EMG activity in the same exercise but may show maximum EMG activity in other exercises.10,15 Our findings confirmed this observation; for example, MVC activity for the external oblique muscle was found in 4 different MVC tasks (Figure 2). The most productive exercise for the dorsal extensor muscles was not a machine-based exercise (as expected) but the prone-lying extension of the whole body from a slight flexed-hip position. One explanation for this finding could be the combination of the subject's stable position and the activation of the whole extensor chain, which facilitated the activity of all the synergistic muscles.
The activity distribution profiles (Figures 3A and B) can be used to estimate the effectiveness of an exercise set in terms of activating the main superficial trunk and hip muscles. However, the high SD ranges in all the EMG data reflect each individual's unique response to these exercises, despite the homogeneity of the subjects' skill levels and familiarity with the exercises. The mean CV of the average activation profiles of 3 selected target muscles ranged from 34.17% (external oblique in lateral-flexion tasks) to 41.18% (lumbar erector spinae in extension tasks) to 43.2% (rectus abdominis in flexion tasks). On average, this variability is comparable with or even lower than that found in other studies (eg, investigations of gait cycles).11 As indicated by this variability, a general conclusion is that a training exercise does not automatically generate a certain stimulus or level of demand for the individual muscle.
Another feature of our study was the analysis of a typical training set for each exercise, including 8 repetitions. One expectation was that fatigue-induced changes of the EMG signal could occur within the sequence of repetitions (eg, increased EMG activity as a result of motor unit recruitment and increased firing frequency).19,20 The statistical analysis of the mean EMG differences between the first and the last repetition revealed that only in 3 exercises did a significant increase in concentric EMG activity occur in the prime movers: the rectus abdominis in the curl-up, hyperextended, and sit-up and the external oblique in the lateral-flexion exercise with fixed legs. Probably as a result of the low initial activation level, typically not exceeding 50% MVC, no significant EMG increase was found for the other muscles and exercises. If training effectiveness is described in terms of strength development, the neuromuscular activation for these tasks may not be high enough, and increased load should be added to achieve an effective neuromuscular innervation higher than 50% MVC.20,21 In practice, this would need to be determined individually, because contrary to the mean tendency, some individual subjects demonstrated steep increases, whereas others did not.
Flexion Exercises
A main finding was the isolation of the abdominal muscle activity in spine-flexion tasks without hip flexion (Figure 3A), which confirms earlier studies.7,8 When hip flexion, such as in the sit-up, was added to spine flexion, the mean flexion activity for the rectus abdominis muscle was unchanged, but the external oblique and rectus femoris muscle activation was significantly higher. McGill et al,22 comparing fine-wire and surface electrodes, found that with an error of about 12%, rectus femoris activity in common flexion tasks can be used to estimate the activity of the deep psoas muscle. Mean rectus femoris flexion activity in our data increased from 4.26% MVC (curl-up) to 32.18% MVC (sit-up), indicating clear involvement of the hip-flexor muscles. However, this finding demonstrated that the abdominal flexors remained the primary activated muscles.
The activation profiles of the averaged repetition cycle (Figure 4) illustrated remarkable differences in the development of the rectus abdominis activity for the curl-up and the sit-up, despite the fact that the mean activity was the same. This difference was mainly due to increased initial velocity in the sit-up exercise, in which the upper body ROM was increased by 60°, but the contraction time of 2 seconds was kept constant. In flexion tasks such as the curl-up and the sit-up, the abdominal muscles are mainly active within the first 30° of flexion.2–4 From the standpoint of muscular training effectiveness and peak activation, our data indicate that the sit-up is the more demanding exercise for both the rectus abdominis and the external oblique muscles due to the increased contraction velocity and the need to accelerate the upper body mass more quickly at the beginning of movement. As Axler and McGill8 showed, this happens at the cost of higher compression forces acting at the lumbar vertebrae. Results from other studies7,23,24 allow for the same conclusion of increased activation in the abdominal muscles in sit-ups, even if a direct comparison is limited due to different test standardization and EMG quantification. Similar to the study of Ekholm et al,23 the mean EMG activity but not the peak activity for the (upper) rectus abdominis was significantly increased when the muscle was prestretched, as in the curl-up, hyperextended.
Lateral-Flexion Exercises
High neuromuscular demand on the external oblique was found in the 2 gymnastic lateral-flexion exercises. The other abdominal and back muscles acted as synergists with a mean activation level between 40% and 60% MVC. The analysis of the average activation profiles for the external oblique muscle resulted in supramaximal EMG values, even if the load itself was clearly submaximal. Typical data published for lateral-flexion tasks range from 40% to 75% MVC, depending on whether static8,25 or dynamic10 exercises were performed. Supramaximal EMGs under submaximal dynamic load have been noted in other studies as well, but the findings were not addressed by the authors.8,14,18,26 The cause of this phenomenon remains unclear. A possible reason could be the incomplete excitation of the motoneurons within the static MVC test trial, despite the fact that similar exercise arrangements were used and the subjects were accustomed to performing at maximum effort. Another cause could be the changing electrode-to-muscle configuration and distance in dynamic vs static contractions, which is thought to influence the validity of static MVCs.1,27–29 Changes in the recruitment scheme, increased cross-talk, metabolic changes occurring in repeated dynamic contractions, and signal-summation effects are other possible sources for supramaximal amplitudes. The estimation of the neuromuscular activation level is clearly limited by these uncertainties. However, because MVC amplitude normalization is mainly a rescaling function, the relative (muscle-specific) comparison of EMG activities among several tasks is not affected and should be the main focus of interpretation.
As compared with lateral-flexion tasks, a comparably low activation of the external oblique muscle was achieved with the cross curl-up exercise (Figure 3A). The increase in mean activity associated with changing the regular curl-up to the cross curl-up was not significant in this study, but other researchers8,10,23,24 have found that rotation added to spine-flexion tasks resulted in a higher external oblique activation. For all lateral-flexion exercises, the main activity took place in the external oblique muscle. Marras and Granata25 investigated isokinetic lateral flexion in the standing position and demonstrated that, after the internal and external oblique muscles, the latissimus dorsi was the most active muscle.
Extension Exercises
Among the back-extension exercises, diagonal hip and shoulder extension and bridging showed comparatively low mean EMG activities of less than 35% MVC. The load and activation demand may not be high enough to produce a stimulus sufficient for strength development in conditioning programs.21 As demonstrated in the mean activity distribution profile (Figure 3B), the diagonal hip and shoulder extension can be characterized as a “whole-body” exercise on a low activation level. Callaghan et al9 found comparable EMG activities for the thoracic and 2 portions of the lumbar erector spinae muscle in this exercise.
Bridging is sometimes misinterpreted as predominantly a hip-extensor exercise. The most active muscle, however, was the erector spinae at the lumbar and thoracic portions, whereas the mean extension activity of the gluteus and semitendinosus and semimembranosus group was less than 14%. The relatively low activation of the gluteus was also found in a patient population studied by Liefring et al.30 Good isolation of the thoracic and lumbar erector spinae muscles was achieved with the kneeling back extension, where a flexed and static hip position facilitated selective activity of the lumbar extensor muscles.
In contrast to this exercise, the whole dorsal extensor chain was activated in the trunk extension, fixed legs and hip extension, fixed trunk. In both tasks, the neuromuscular activity was higher for the spine-extensor muscles than for the hip-extensor muscles, indicating that these tasks were mainly “back-training” exercises. Callaghan et al9 reported peak EMG activities of the erector spinae between 45% and 60% MVC in the trunk extension, fixed legs.
A direct comparison of these movements demonstrated a nonsignificant trend for the fixed-trunk version to enhance erector spinae muscle activity and diminish hip-extensor activity. As shown in Figure 4, peak erector spinae activity for combined spine- and hip-extension movement is located at the end of the extension period, when the body segments provide the highest lever arm. From the standpoint of training effectiveness, the last 25% of the extension cycle is the most productive part of movement. Callaghan et al9 demonstrated that EMG activity is increased when true hyperextension of the spine and hip joint is performed. In 2 other studies,6,30 near-maximal EMG activity was found in the lumbar erector spinae portion when unilateral and bilateral straight-leg lifting was performed to achieve prone hyperextension.
The increase in erector spinae activity with increasing extension is also reported for the combined back- and hip-extension task in the standing position.28,31 In a comparison study between kneeling and standing back and hip extension, Gallagher31 demonstrated that the angle-specific activation of the erector spinae muscle is strongly influenced by the hip and pelvis position and rotation. Many investigators have used seated or standing back- and hip-extension and lifting tasks without measuring the activity of the hip-extensor muscles.17,26,28,31–34 Our findings for combined spine- and hip-extension exercises, such as trunk extension with fixed trunk or fixed legs, demonstrated synchronized activation of all dorsal chain muscles, indicating the need to monitor all of them when back-extension tasks are studied. As shown for the kneeling trunk-extension exercise, lumbar and thoracic erector spinae activity could be isolated for the hip extensors' activity when the hip was fixed in a flexed position.
CONCLUSIONS
Despite movement standardization, the EMG data in our study demonstrated remarkable intersubject variability. Even if some of this variation was due to the limitations of the MVC normalization concept, it outlines the range of individual responses and training effectiveness for a given exercise. Within the limitations of the type and size (n = 10) of the population investigated, neuromuscular innervation trends for trunk-training exercises can nevertheless be summarized.
· With pure spine-flexion exercises, such as the curl-up, good isolation of the abdominal muscles can be achieved. The mean EMG activity slightly increased through additional prestretch or rotation. Combined spine- and hip-flexion exercises, such as the sit-up, increased the EMG activity of the abdominal muscles, mainly due to the changes in velocity and ROM.
· Very high activation of the external oblique muscle took place in lateral-flexion exercises in the side-bending position. The lumbar erector spinae and the rectus abdominis muscles showed distinctive coactivation within these tasks.
· Double-supported back-extension exercises, such as diagonal hip and shoulder extension or bridging, elicited moderate mean activity in the dorsal-extensor muscles. Only low hip-extensor activity was achieved with bridging. Erector spinae muscle activity was effectively increased during prone-lying upper body versus lower body movements.
· Activation of the hip-extensor and spine-extensor muscles was closely coupled in these combined spine- and hip-extension exercises, but the main neuromuscular activity was still located in the back muscles. The spine extensors can be isolated with exercises based on a fixed hip in flexed position and segmental spine extension.
REFERENCES
- 1.De Luca CJ. The use of surface electromyography in biomechanics. J Appl Biomech. 1997;13:135–163. [Google Scholar]
- 2.Godfrey KE, Kindig LE, Windell EJ. Electromyographic study of duration of muscle activity in sit-up variations. Arch Phys Med Rehabil. 1977;58:132–135. [PubMed] [Google Scholar]
- 3.Halpern AA, Bleck EB. Sit-up exercises: an electromyographic study. Clin Orthop. 1979;145:172–178. [PubMed] [Google Scholar]
- 4.Ricci B, Marchetti M, Figura F. Biomechanics of sit-up exercises. Med Sci Sports Exerc. 1981;13:54–59. [PubMed] [Google Scholar]
- 5.Andersson EA, Oddsson L, Grundström H, Thorstensson A. The role of the psoas and iliacus muscles for stability and movement of the lumbar spine, pelvis and hip. Scand J Med Sci Sports. 1995;5:10–16. doi: 10.1111/j.1600-0838.1995.tb00004.x. [DOI] [PubMed] [Google Scholar]
- 6.Andersson EA, Oddsson L, Grundström H, Nilsson J, Thorstensson A. EMG activities of the quadratus lumborum and erector spinae muscles during flexion-relaxation and other motor tasks. Clin Biomech. 1996;7:392–400. doi: 10.1016/0268-0033(96)00033-2. [DOI] [PubMed] [Google Scholar]
- 7.Andersson EA, Nilsson J, Ma Z, Thorstensson A. Abdominal and hip flexor muscle activation during various training exercises. Eur J Appl Physiol Occup Physiol. 1997;75:115–123. doi: 10.1007/s004210050135. [DOI] [PubMed] [Google Scholar]
- 8.Axler CT, McGill SM. Low back loads over a variety of abdominal exercises: searching for the safest abdominal challenge. Med Sci Sports Exerc. 1997;6:804–810. doi: 10.1097/00005768-199706000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Callaghan JP, Gunning JL, McGill SM. The relationship between lumbar spine load and muscle activity during extensor exercises. Phys Ther. 1998;78:8–18. doi: 10.1093/ptj/78.1.8. [DOI] [PubMed] [Google Scholar]
- 10.Juker D, McGill S, Kropf P, Steffen T. Quantitative intramuscular myoelectric activity of lumbar portions of psoas and the abdominal wall during a wide variety of tasks. Med Sci Sports Exerc. 1998;30:301–310. doi: 10.1097/00005768-199802000-00020. [DOI] [PubMed] [Google Scholar]
- 11.Yang JF, Winter DA. Electromyographic amplitude normalization methods: improving their sensitivity as diagnostic tools in gait analysis. Arch Phys Med Rehabil. 1984;65:517–521. [PubMed] [Google Scholar]
- 12.Komi PV. Relationship between muscle tension, EMG and velocity of contraction under concentric and eccentric work. In: Desmedt JE, editor. New Developments in Electromyography and Clinical Neurophysiology. Vol 1. Basel, Switzerland: Karger; 1973. pp. 596–606. [Google Scholar]
- 13.Redfern M. Functional muscle: effects on electromyographic output. In: Soderberg GL, editor. Selected Topics in Surface Electromyography for the Use in the Occupational Setting: Expert Perspectives. Cincinnati, OH: US Department of Health and Human Services, Public Health Service; 1992. pp. 104–120. [Google Scholar]
- 14.De Looze MP, Toussaint HM, van Dieen JH, Kemper HCG. Joint moments and muscle activity in the lower extremities and lower back in lifting and lowering tasks. J Biomech. 1993;26:1067–1076. doi: 10.1016/s0021-9290(05)80006-5. [DOI] [PubMed] [Google Scholar]
- 15.McGill SM. The mechanics of torso flexion: situps and standing dynamic flexion maneuvers. Clin Biomech. 1995;4:184–192. doi: 10.1016/0268-0033(95)91396-v. [DOI] [PubMed] [Google Scholar]
- 16.Miller MI, Medeiros JM. Recruitment of internal oblique and transversus abdominis muscles during the eccentric phase of the curl-up exercise. Phys Ther. 1987;67:1213–1217. doi: 10.1093/ptj/67.8.1213. [DOI] [PubMed] [Google Scholar]
- 17.Toussaint HM, de Winter AF, de Haas Y, de Looze MP, van Dieen JH, Kingma I. Flexion relaxation during lifting: implications for torque production by muscle activity and tissue strain at the lumbo-sacral joint. J Biomech. 1995;28:199–210. doi: 10.1016/0021-9290(94)00051-5. [DOI] [PubMed] [Google Scholar]
- 18.Shields RK, Heiss DG. An electromyographic comparison of abdominal muscle synergies during curl and double straight leg lowering exercises with control of the pelvic position. Spine. 1997;22:1873–1879. doi: 10.1097/00007632-199708150-00012. [DOI] [PubMed] [Google Scholar]
- 19.Bigland-Ritchie B, Woods JJ. Changes in muscle contractile properties and neural control during human muscular fatigue. Muscle Nerve. 1984;7:691–699. doi: 10.1002/mus.880070902. [DOI] [PubMed] [Google Scholar]
- 20.Häkkinen K, Kauhanen H, Komi PV. Effects of fatiguing loading with a variable resistance equipment on neural activation and force production of the knee extensor muscles. Electromyogr Clin Neurophysiol. 1988;28:79–87. [PubMed] [Google Scholar]
- 21.Enoka RJ. Neuromechanical Basis of Kinesiology. 2nd ed. Champaign, IL: Human Kinetics; 1994. pp. 303–349. [Google Scholar]
- 22.McGill S, Juker D, Kropf P. Appropriately placed surface EMG electrodes reflect deep muscle activity (psoas, quadratus lumborum, abdominal wall) in the lumbar spine. J Biomech. 1996;29:1503–1507. doi: 10.1016/0021-9290(96)84547-7. [DOI] [PubMed] [Google Scholar]
- 23.Ekholm J, Arborelius U, Fahlcrantz A, Larsson AM, Mattsson G. Activation of abdominal muscles during some physiotherapeutic exercises. Scand J Rehabil Med. 1979;11:75–84. [PubMed] [Google Scholar]
- 24.Noble L. Effects of various types of situps on IEMG of the abdominal musculature. J Hum Mov Stud. 1981;7:124–130. [Google Scholar]
- 25.Marras WS, Granata KP. Spine loading during trunk lateral bending motions. J Biomech. 1997;30:697–703. doi: 10.1016/s0021-9290(97)00010-9. [DOI] [PubMed] [Google Scholar]
- 26.Ross EC, Parnianpour M, Martin D. The effects of resistance level on muscle coordination patterns and movement profile during trunk extension. Spine. 1993;18:1829–1838. doi: 10.1097/00007632-199310000-00019. [DOI] [PubMed] [Google Scholar]
- 27.Le Veau B, Andersson GBJ. Output forms: data analysis and applications. In: Soderberg GL, editor. Selected Topics in Surface Electromyography for the Use in the Occupational Setting: Expert Perspectives. Cincinnati, OH: US Department of Health and Human Services. Public Health Service; 1992. pp. 69–102. [Google Scholar]
- 28.Mirka GA. The quantification of EMG normalization error. Ergonomics. 1991;34:343–352. doi: 10.1080/00140139108967318. [DOI] [PubMed] [Google Scholar]
- 29.Vakos JP, Nitz AJ, Threlkeld AJ, Shapiro R, Horn T. Electromyographic activity of selected trunk and hip muscles during a squat lift: effect of varying the lumbar posture. Spine. 1994;19:687–695. doi: 10.1097/00007632-199403001-00008. [DOI] [PubMed] [Google Scholar]
- 30.Liefring V, Hinz K, Seidel W, Conradi E. Objektivierung der Muskelaktivität bei krankengymnastischen Bewegungsabläufen mit Mehrkanalelektromyographie. Phys Rehabil Kur Med. 1991;1:33–37. [Google Scholar]
- 31.Gallagher S. Trunk extension strength and muscle activity in standing and kneeling postures. Spine. 1997;22:1864–1872. doi: 10.1097/00007632-199708150-00011. [DOI] [PubMed] [Google Scholar]
- 32.Dolan P, Mannion AF, Adams MA. Passive tissues help the back muscles to generate extensor moments during lifting. J Biomech. 1994;27:1077–1085. doi: 10.1016/0021-9290(94)90224-0. [DOI] [PubMed] [Google Scholar]
- 33.Granata KP, Marras WS. An EMG-assisted model of loads on the lumbar spine during asymmetric trunk extensions. J Biomech. 1993;26:1429–1438. doi: 10.1016/0021-9290(93)90093-t. [DOI] [PubMed] [Google Scholar]
- 34.Lavender S, Trafimow J, Andersson GBJ, Mayer RS, Chen IH. Trunk muscle activation: the effects of torso flexion, movement direction, and moment magnitude. Spine. 1994;19:771–778. [PubMed] [Google Scholar]