Abstract
Objective:
To test the influence of cooling on proprioceptive acuity as reflected in the ability to discriminate weights.
Design and Setting:
Participants were trained to perform a weight-discrimination task. Their ability to correctly report small increments in weight was compared before and after local cooling (a 20-minute application of a crushed-ice pack) of the quadriceps muscle group. Data were collected at a university research laboratory.
Subjects:
Twenty young, physically active adults (undergraduate students; 14 men, 6 women; mean age, 22.1 ± 2.6 years).
Measurements:
We calculated overall performance in the weight-discrimination task (percentage of discrimination correct) for each participant to estimate the differential threshold (ie, minimal increment in weight that yields a probability of 75% correct responses).
Results:
Before local cooling, participants discriminated increments in the order of 4% to 10% from the standard weight (mean threshold, 0.17 ± 0.06 kg). After local cooling, the discriminative performance remained, on average, very similar to that seen before cooling (mean threshold, 0.17 ± 0.08 kg; paired t test: t = 0.24, P = .81). Only a small group of participants (n = 5) showed evidence of a decreased ability to discriminate weight after cooling.
Conclusions:
The perception of force signals required for weight discrimination does not appear to be affected by local cooling of the quadriceps muscle group. This finding provides additional evidence for the relative safety of cold applications and their effect on proprioceptive perceptual abilities.
Keywords: cold therapy, weight perception, sensory discrimination
In the context of sports therapy, ice is frequently used to treat minor acute musculoskeletal injuries. Although ice is known to be effective in decreasing painful and tactile sensations, its effect on proprioceptive abilities has received comparatively little attention. La Riviere and Osternig1 evaluated the effect of brief (5-minute) and prolonged (20-minute) cold-water immersion on ankle proprioception and concluded that cooling does not alter position sense. Thieme et al2 reported similar findings for the knee joint after a 20-minute ice application. Thus, perception of static joint positions seems to be preserved after cooling. The ability to sense joint position, however, is only one of the perceptual attributes of the proprioceptive system, which also includes the ability to sense movement (amplitude and angular velocity) and to perceive force and weight.3 The latter ability is particularly important both from historical and perceptual points of view. Weber in 1834 was the first to fully appreciate the importance of weight perception in relation to what he called the “muscular sense.” Weber's seminal observation that weight discrimination was far more accurate when objects were actively lifted instead of being passively applied on skin provided the first evidence that signals from contracting muscles participate in weight perception.4 At that time, however, it was not clear if the muscular sense, and therefore weight perception, was derived from signals of peripheral origin or from sensory signals arising centrally via corollaries of the descending motor commands producing the lifting action. More recent investigations have helped to clarify the issue and confirmed that sensory signals of both central and peripheral origins contribute to our ability to perceive force and weights.4
Given the acute sensitivity of the human proprioceptive system to changes in weight (typically, normal subjects can reliably discriminate a 5% to 10% change in weight with active lifting movements5,6), we thought that weight discrimination would be a good test to determine the influence of cooling on proprioceptive acuity. The purpose of our study, therefore, was to test whether the ability to perceive small differences in weight (ie, proprioceptive acuity) remained accurate after local cooling of the quadriceps muscle group.
METHODS
Participants
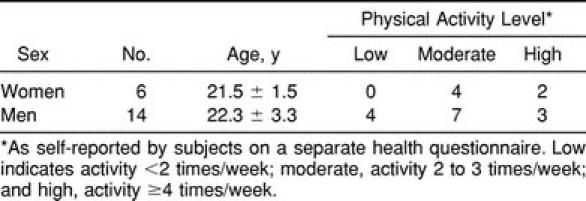
Twenty young, healthy adults (mean age, 22.1 ± 2.6 years) were recruited from among the population of undergraduate students at the Faculty of Health Sciences, University of Ottawa. All participants were physically active (Table 1), and some were even engaged in high-level activity (1 participant was a player on the Canadian women's Olympic hockey team). None reported a previous history of knee injuries or sensory dysfunction in the right lower extremity. The study's procedures were approved by the University Human Research Ethics Board, and subjects gave their informed consent.
Table 1.
Participants' Characteristics
Materials
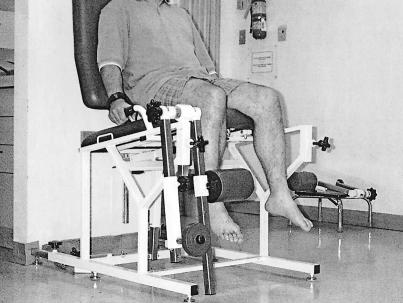
The ability of participants to discriminate weights was tested on a conventional leg exercise table (Model 2400, Midland Co, Columbia, SC) equipped with a lever system that allowed for loading of free weights (Figure 1). The actual weight of the table's right lever system unloaded (2.50 kg measured separately on a precision numeric scale) was chosen as the standard. Pilot testing indicated that a standard weight of 2.50 kg was appropriate to prevent fatigue during testing because participants had to perform many repeated lifting movements. A set of metric metal weights (0.11, 0.28, 0.40, and 0.50 kg, Elgin Co, Elgin, IL) was used to gradually increase the mass of the standard weight. These comparison weights corresponded to increments of 4% (2.61 kg), 11% (2.78 kg), 16% (2.90 kg), and 20% (3 kg) from the standard. This set of comparison weights covered the range of human capacities for weight discrimination along a continuum from easy (16% and 20% changes) to increasingly difficult (4% and 10% changes) discrimination.
Figure 1.
The participant's starting position on the exercise table with the 0.50-kg increment weight on the lever. Note that the standard weight corresponded to the weight of the lever unloaded (2.50 kg).
Weight-Discrimination Task
Precooling
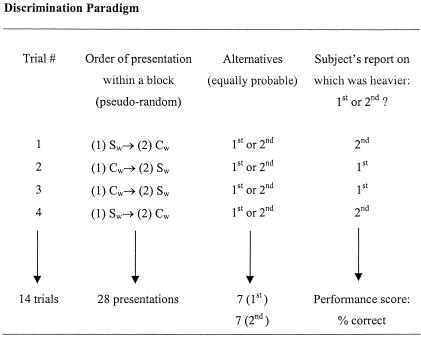
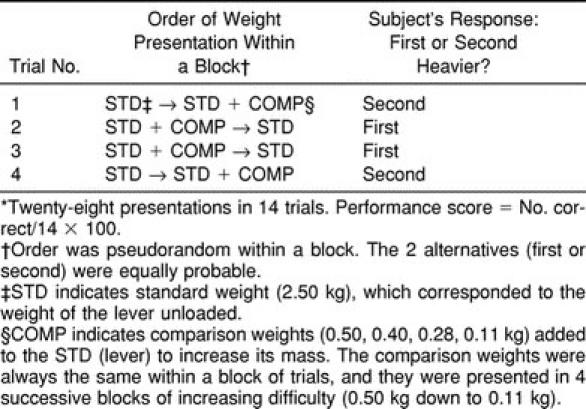
As shown in Figure 1, the task was performed with the participant comfortably seated on the exercise table with the hips flexed to approximately 100° and the knee resting at 90°. The leg pad was adjusted in height approximately 5 cm above the medial malleolus of the right leg. Participants were then instructed regarding the task. They were told that it consisted of active-lifting movements in order to compare weights with the right leg. Participants were free to choose any movement ranges (from 90° to 180°), speeds, and modes of contraction (concentric or eccentric) they deemed appropriate to estimate the weights. To prevent any bias, participants were not informed that the standard weight corresponded to the weight of the lever unloaded. In addition, to avoid pressure cues, the lever was always pulled away from the participant's leg for weight loading and unloading. Participants were then blindfolded and provided with a series of practice trials, which consisted of 5 easy discriminations (0.50-kg increments) and 5 more difficult discriminations (0.28-kg increments). During practice trials, feedback was given on discrimination performance. Once participants felt comfortable with the task, the formal testing session began. Participants remained blindfolded and were fitted with earplugs to eliminate any auditory cues. The discrimination protocol is described in Figure 2. Briefly, it consisted of a 2-alternative, forced choice (2-AFC) procedure with each trial consisting of 2 weight presentations: the standard weight (2.50 kg) and a comparison weight (eg, a 0.50-kg increment). After receiving a tactile cue (a tap on the knee), participants lifted each weight successively and reported which in the sequence (the first or second weight) felt heavier. The order of presentation (standard versus comparison) was pseudorandom, and the 2 alternatives were equally probable within a block of 14 trials (Table 2). As usual in discrimination paradigms,7,8 the comparison weights were presented in 4 successive blocks of increasing difficulty (ie, 0.50-kg increments down to 0.11-kg increments). Although no restriction was imposed on the lifting movement, most participants chose an up-and-down strategy to judge the applied weight, moving up the lever slowly near full extension (concentric mode), then returning slowly to the starting position (eccentric mode). No feedback was provided on the discrimination performance during or after formal testing. The whole precooling procedure was usually completed within 30 minutes, the task itself taking 10 to 15 minutes to complete.
Figure 2.
Protocol used for weight discrimination. Sw indicates standard weight (2.50 kg), which corresponded to the weight of the lever unloaded. Cw indicates comparison weights (0.50-, 0.40-, 0.28-, and 0.11-kg increments) that were added separately to the standard (lever) to increase its mass. Note that the comparison weight was always the same within a block of trials and that the blocks were presented in an overall sequence of increasing difficulty (ie, first block, 0.50 kg; second block, 0.40 kg; third block, 0.28 kg; and fourth block, 0.11 kg).
Table 2.
Discrimination Paradigm Used for the Weight-Discrimination Task*
Cooling
Participants were placed on a bed with the right knee in slight flexion and resting on a pillow. Before ice application, the right thigh-skin sensibility was briefly tested by checking response to pinprick. The ice was applied in the form of crushed ice in a moist towel over the anterior aspect of the thigh for 20 minutes to obtain effective cooling of the quadriceps muscle belly. This method of cooling has been shown to be effective in decreasing intramuscular temperature.9,10 The ice application was adjusted for each participant so that it covered at least two thirds of the anterior thigh, excluding the most distal (patellar) and proximal (groin) areas. After the 20 minutes had elapsed, the ice pack was removed and the skin was reinspected. All participants reported diminished sensation and a decreased response to pinprick in the cooled area.
Postcooling
After the cooling procedure, participants were rapidly returned (usually within 2 minutes) to the exercise table to be tested again with the weight-discrimination task. As in the precooling procedure, testing took 10 to 15 minutes to complete.
Data Analysis
Proprioceptive acuity was determined by calculating for each participant the performance in the weight-discrimination task (ie, the number of correct discriminations for each comparison weight). A discrimination function was then constructed for each participant by plotting performance values (in percentages) against increments in weight. Although the discrimination function provides a description of the discriminative behavior, it is convenient to extract a single representative value for this capacity. For this purpose, we used the differential threshold, which, by convention in a 2-AFC procedure, corresponds to the minimal increment that yields a probability of 75% correct responses (ie, midway between chance and perfection).7,8 The differential threshold was computed by linear interpolation between the performance values on either side of the 0.75 value.7 In some instances, performance was greater than or equal to the 75% level even with the lightest increment in weight (ie, 0.11 kg). In these cases, the threshold was set at 0.11 kg for statistical purposes. Precooling and postcooling differential thresholds were compared using a paired t test at the P < 0.05 level.
RESULTS
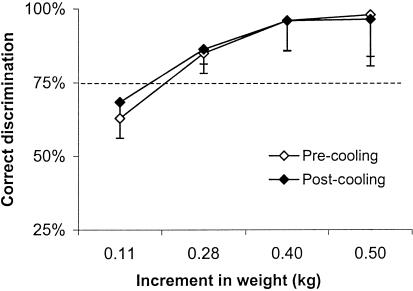
Figure 3 demonstrates the mean performance in the weight-discrimination task before and after local cooling. Before cooling, participants had no difficulty discriminating the 2 largest comparison weights (the 0.50- and 0.40-kg increments) from the standard. For the 2 other comparison weights, the discrimination performance decreased gradually and fell below the 75% level for the lightest increment (0.11 kg). Yet some participants (n = 6) were able to provide reliable reports of weight change (performance greater than or equal to the 75% level), even for the 0.11-kg increment. Overall, the differential threshold for weight discrimination before cooling represented, on average, a 6.8% increase from the standard weight (mean, 0.17 ± 0.06 kg). After cooling, the discriminative performance was, on average, very similar to that seen before cooling (Figure 3), and the differential threshold remained stable (mean, 0.17 ± 0.08 kg; t = 0.24; P = 0.81).
Figure 3.
Performance in the weight-discrimination task before and after cooling. Each value represents the mean performance of all participants (n = 20) for each increment in weight with the associated SD in one direction. The dotted line indicates the 75% correct discrimination level, which corresponds by convention to the differential threshold.
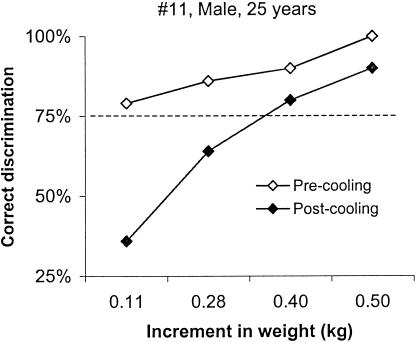
Although the ability to discriminate weights was unaffected by cooling in most participants, a small number of individuals (n = 5) experienced a decline in performance after cooling (ie, individual threshold values increased by a factor of 1.5 to 3.5 as compared with precooling values). Figure 4 displays the results for such an individual. Before cooling, this participant (a moderately active 25-year-old man) had no difficulty discriminating large and small increments in weight (threshold, 0.11 kg). However, after cooling, his discriminative ability greatly deteriorated, as evident in the major shift of the discrimination function (postcooling threshold, 0.40 kg). Such a decline in weight discrimination after cooling was not particular to a sex (4 men, 1 woman) or to a level of physical activity (4 were moderately active, 1 was highly active).
Figure 4.
An individual whose discriminative performance was affected after cooling. The representation is similar to the representation in Figure 3
DISCUSSION
Our study has shown that proprioceptive acuity in the quadriceps muscle, as reflected in the ability to perceive differences in weight, is preserved after a prolonged ice application. Before cooling, participants were able to reliably discriminate 4% to 10% increments in weight (threshold range, 0.11 to 0.26 kg), a range comparable to that reported in previous studies on weight discrimination.5,6 After cooling, the perception of force signals generated during weight lifting remained accurate in most participants. In the following discussion, we will first address the issue of effectiveness of cooling methods as they pertain to proprioception. We will then discuss the possible sensory mechanisms underlying the current and previous observations that cooling has no effect on proprioceptive abilities.
The present finding that cooling did not affect the ability to discriminate weights is in agreement with the findings of previous studies that reported no change in the sense of position in the lower limb.1,2 Thus, proprioceptive abilities appear to be relatively insensitive to the cooling effect. This finding raises the question of why proprioception is unaffected, whereas thresholds for cutaneous sensations rise sharply after local cooling.11 One obvious possibility is that the common methods of cooling are simply not effective in decreasing the temperature in deep tissue, where important proprioceptors are located. Indeed, studies have shown that the degree of cooling achieved in deep tissues varies widely depending upon the method used (eg, ice packs versus gel packs or cold-water immersion).12 We used a conventional method to cool the quadriceps muscle (crushed-ice pack for 20 minutes). Although we did not monitor tissue temperature, other authors have reported significant reductions in intramuscular temperature using similar applications.9,10,13 Thus, we can reasonably assume that our cooling method was effective in decreasing muscle temperature. The degree of cooling achieved in each individual may have been different, however, due to uncontrolled factors such as thickness of adipose tissue. Nevertheless, all participants reported diminished sensation in the cooled area (response to pinprick) after the application and exhibited the usual objective signs of tissue cooling such as intense skin redness.
Another related issue pertains to the persistence of the cooling effect. As stated in the Methods section, the participants were tested within 2 minutes after application, and testing took 10 to 15 minutes to complete. Therefore, one may argue that by the time the testing procedure ended, the temperature had already returned to its preapplication levels. Although we cannot rule out this possibility, a recent study by Merrick et al9 showed that an ice wrap applied over the anterior thigh for 30 minutes produced significant cooling in deep tissues (2 cm) that persisted up to 20 minutes after application. Thus, it is very unlikely that all of the cooling-induced effect had completely vanished by the time the postcooling testing procedure was administered.
Whatever the issues about the depth and duration of temperature changes, cooling is known to produce marked and persistent slowing of peripheral nerve conduction. For example, after cooling the calf muscles for 20 minutes, Halar et al13 reported an average reduction of 7.4°C in skin temperature with corresponding drops in sural and tibial nerve conduction velocity of 11.2 and 6.4 m·s−1, respectively. The H-reflex latency, which reflects conduction in proprioceptive afferents from muscle spindles, increased, on average, by 5.3 milliseconds. Thus, peripheral signals of cutaneous and muscle origin were very likely (if not certainly) reduced after cooling. A reduction in skin afferents, although critical for tactile and pain sensations, is of less consequence for proprioceptive abilities. Indeed, skin mechanoreceptors contribute little to proprioceptive acuity, at least in the larger joints of the lower extremity.14,15 Alternatively, any reduction in muscle afferents could be more detrimental for proprioceptive acuity because signals from muscle spindles appear to be critical to our ability to sense joint position and movement.15–17 As previously noted, the ability to sense joint position is apparently unaffected after cooling. La Riviere and Osternig1 suggested that inputs from joint receptors are probably able to compensate for cold-induced reductions in skin and muscle afferents. This proposal is unlikely, however, because joint receptors are only activated at the extremes of the joint range during passive movement,14 a property that makes them poor candidates to signal joint position. Another possibility resides in the differential response of primary and secondary spindle endings to changes in muscle temperature.18,19 Mense18 studied the effect of temperature on cat muscle spindles and reported that primary endings were uniformly depressed in a cold muscle (approximately 29°C), whereas most secondary endings showed the reverse effect (ie, increased response to stretch). Whereas the reduced outflow from primary endings is consistent with reductions in tendon reflex amplitude reported in humans,20 the increased outflow from secondary spindle endings may well explain the preserved ability to sense joint position after cooling, as previously noted. Indeed, secondary endings have been implicated in the perception of absolute joint angle because they are thought to function as length detectors.15
Another class of proprioceptive afferents that is particularly important with regard to the present findings is tendon organs. We deliberately restricted the cold application to the anterior thigh to reproduce situations often encountered in sports therapy (eg, treating a muscle bruise). The application, therefore, did not cover the joint or the quadriceps tendon. The major role ascribed to tendon organs in the genesis of sensations of force and effort4 may explain why weight discrimination was preserved in most participants, since tendon organs were, presumably, less affected by cooling. Evidence in animals, however, suggests that afferents from tendon organs are not markedly affected by changes in muscle temperature.18 Yet, there remains the possibility that, even with a profound reduction in afferents from muscle spindles or tendon organs, weight discrimination can still be performed on the basis of corollary discharges associated with the active-lifting movements.4,21 Psychophysical experiments have shown that when peripheral feedback is reduced or perturbed, individuals tend to rely more on sensory information generated centrally (via corollaries of descending commands) than on signals arising peripherally to make judgements about force and weight.4,22 Thus, participants may have compensated for the reduction in peripheral signals by estimating the degree of efferent activity (via corollary discharges) necessary to lift the weight. Some participants did experience a decline in their ability to discriminate weight after cooling (Figure 4). The discrimination performance of those participants may have relied more on signals arising peripherally than on signals arising centrally, even if the participants were not necessarily aware of this effect. Interestingly, 2 of these participants made comments such as “my leg felt heavier” or “my quad was numb” after completing the postcooling testing, suggesting that they were more focused on sensations coming from their cooled muscle.
In conclusion, our study provides additional evidence that proprioceptive acuity in the quadriceps muscle group remains largely unaffected after prolonged ice application to the thigh. The fact that the sensory mechanisms underlying the perception of limb position1,2 and sensations of force remained operational after cooling suggests that motor performance should not be affected. Indeed, there is evidence that cryotherapy has only minor consequences for motor performance.23 Thus, a rapid return to play after ice therapy may not be necessarily detrimental for the athlete. Of course, other components of motor performance (eg, strength and flexibility) might be affected after ice therapy,23,24 and health care professionals, therefore, must fully evaluate the conditions and circumstances before returning an athlete to action.
REFERENCES
- 1.La Riviere J, Osternig LR. The effect of ice immersion on joint position sense. J Sport Rehabil. 1994;3:58–67. [Google Scholar]
- 2.Thieme HA, Ingersoll CD, Knight KL, Ozmun JC. Cooling does not affect knee proprioception. J Athl Train. 1996;31:8–11. [PMC free article] [PubMed] [Google Scholar]
- 3.Jones LA. Peripheral mechanisms of touch and proprioception. Can J Physiol Pharmacol. 1994;72:484–487. doi: 10.1139/y94-071. [DOI] [PubMed] [Google Scholar]
- 4.Jones LA. Perception of force and weight: theory and research. Psychol Bull. 1986;100:29–42. [PubMed] [Google Scholar]
- 5.Brodie EE, Ross HE. Sensorimotor mechanisms in weight discrimination. Percept Psychophys. 1984;36:477–481. doi: 10.3758/bf03207502. [DOI] [PubMed] [Google Scholar]
- 6.Ross HE, Brodie EE. Weber fractions for weight and mass as a function of stimulus intensity. Q J Exp Psychol A. 1987;39:77–88. doi: 10.1080/02724988743000042. [DOI] [PubMed] [Google Scholar]
- 7.Johnson KO. Sensory discrimination: decision process. J Neurophysiol. 1980;43:1771–1792. doi: 10.1152/jn.1980.43.6.1771. [DOI] [PubMed] [Google Scholar]
- 8.Bonnet C. Manuel Pratique de Psychophysique. Paris, France: Armand Collin; 1986. p. 254. [Google Scholar]
- 9.Merrick MA, Knight KL, Ingersoll CD, Potteiger JA. The effects of ice and compression wraps on intramuscular temperatures at various depths. J Athl Train. 1993;28:236–245. [PMC free article] [PubMed] [Google Scholar]
- 10.Myrer JW, Measom G, Fellingham GW. Temperature changes in the human leg during and after two methods of cryotherapy. J Athl Train. 1998;33:25–29. [PMC free article] [PubMed] [Google Scholar]
- 11.Bugaj R. The cooling, analgesic, and rewarming effects of ice massage on localized skin. Phys Ther. 1975;55:11–19. doi: 10.1093/ptj/55.1.11. [DOI] [PubMed] [Google Scholar]
- 12.McMaster WC, Liddle S, Waugh TR. Laboratory evaluation of various cold therapy modalities. Am J Sports Med. 1978;6:291–294. doi: 10.1177/036354657800600513. [DOI] [PubMed] [Google Scholar]
- 13.Halar EM, De Lisa JA, Brozovich FV. Nerve conduction velocity: relationship of skin, subcutaneous and intramuscular temperatures. Arch Phys Med Rehabil. 1980;61:199–203. [PubMed] [Google Scholar]
- 14.Clark FJ, Horch KW, Bach SM, Larson GF. Contributions of cutaneous and joint receptors to static knee-position sense in man. J Neurophysiol. 1979;42:877–888. doi: 10.1152/jn.1979.42.3.877. [DOI] [PubMed] [Google Scholar]
- 15.Matthews PB. Proprioceptors and their contribution to somatosensory mapping: complex messages require complex processing. Can J Physiol Pharmacol. 1988;66:430–438. doi: 10.1139/y88-073. [DOI] [PubMed] [Google Scholar]
- 16.McCloskey DI, Gandevia SC. Role of inputs from skin, joints and muscles and of corollary discharges, in human discriminatory task. In: Gordon G, editor. Active Touch: The Mechanisms of Recognition of Object by Manipulation. Oxford, England: Pergamon Press; 1978. pp. 177–188. [Google Scholar]
- 17.Clark FJ, Burgess RC, Chapin JW, Lipscomb WT. Role of intramuscular receptors in the awareness of limb position. J Neurophysiol. 1985;54:1529–1540. doi: 10.1152/jn.1985.54.6.1529. [DOI] [PubMed] [Google Scholar]
- 18.Mense S. Effects of temperature on the discharges of muscle spindles and tendon organs. Pflugers Arch. 1978;374:159–166. doi: 10.1007/BF00581297. [DOI] [PubMed] [Google Scholar]
- 19.Michalski WJ, Seguin JJ. The effects of muscle cooling and stretch on muscle spindle secondary endings in the cat. J Physiol (Lond) 1975;253:341–356. doi: 10.1113/jphysiol.1975.sp011193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bell KR, Lehmann JF. Effect of cooling on H- and T-reflexes in normal subjects. Arch Phys Med Rehabil. 1987;68:490–493. [PubMed] [Google Scholar]
- 21.McCloskey DI, Gandevia S, Potter EK, Colebatch JG. Muscle sense and effort: motor commands and judgments about muscular contractions. Adv Neurol. 1983;39:151–167. [PubMed] [Google Scholar]
- 22.Gandevia SC, McCloskey DI, Potter EK. Alterations in perceived heaviness during digital anaesthesia. J Physiol (Lond) 1980;306:365–375. doi: 10.1113/jphysiol.1980.sp013402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Evans TA, Ingersoll C, Knight KL, Worrell T. Agility following the application of cold therapy. J Athl Train. 1995;30:231–234. [PMC free article] [PubMed] [Google Scholar]
- 24.Cornwall MW. Effect of temperature on muscle force and rate of muscle force production in men and women. J Orthop Sports Phys Ther. 1994;20:74–80. doi: 10.2519/jospt.1994.20.2.74. [DOI] [PubMed] [Google Scholar]