Abstract
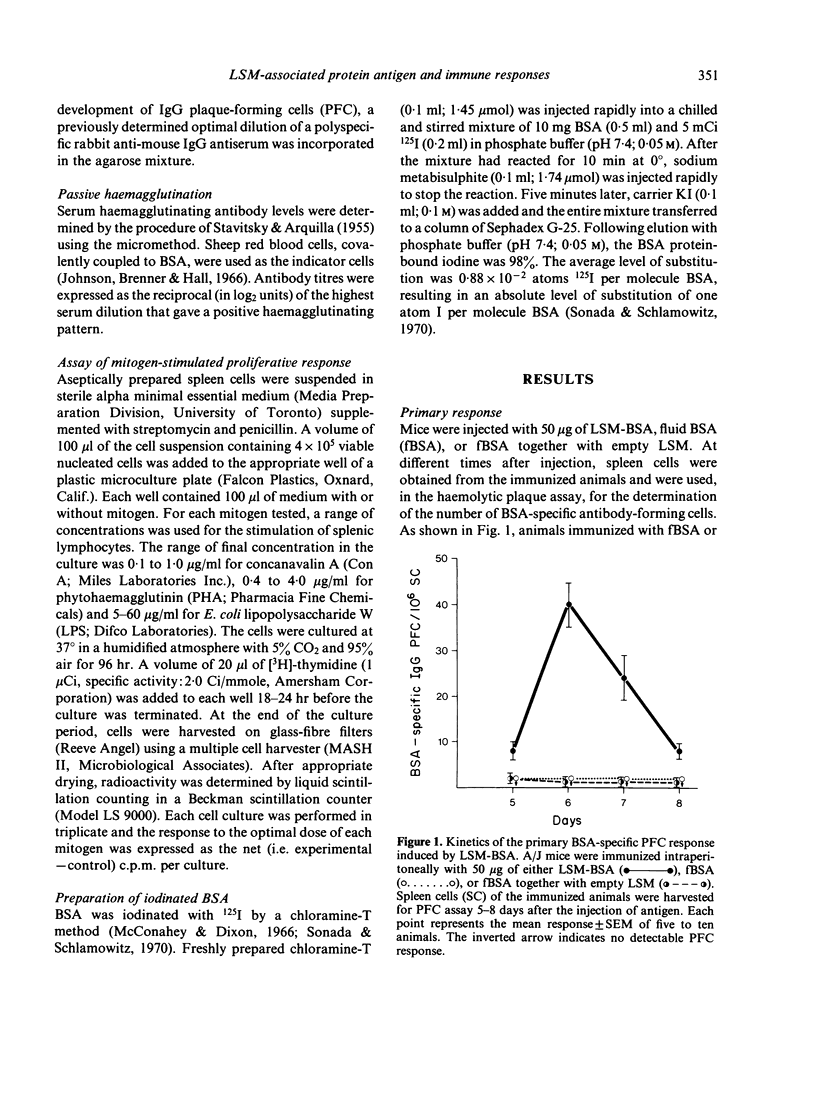
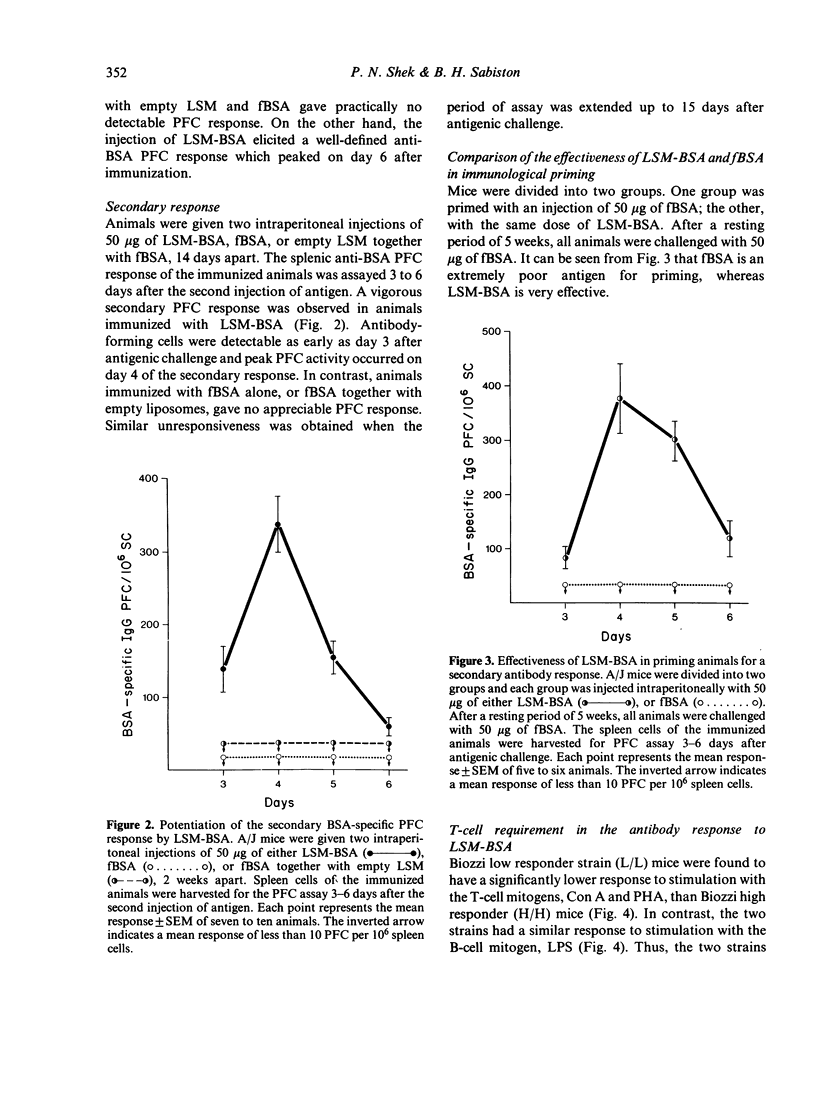
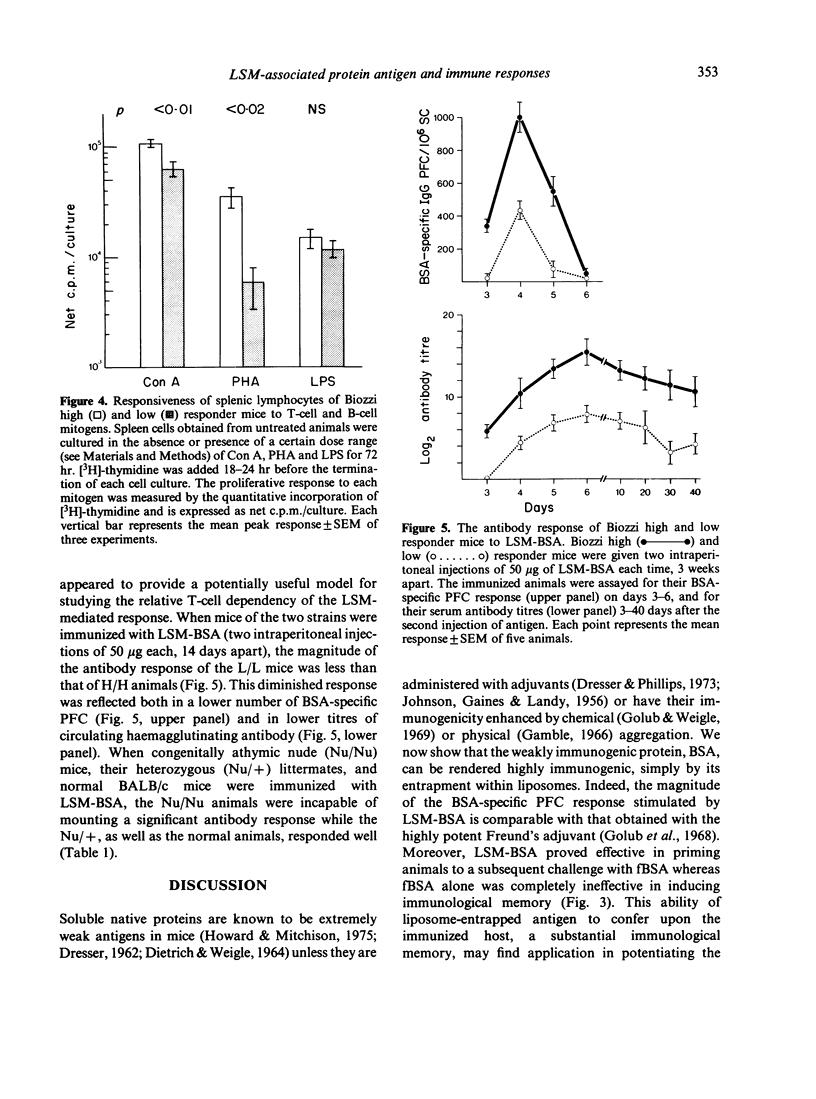
Mice, immunized with liposome-associated bovine serum albumin (LSM-BSA), showed a significantly higher BSA-specific plaque-forming cell (PFC) response than did mice injected with fluid BSA (fBSA). Physical association between the liposome carrier and the protein antigen is imperative for potentiating the PFC response, since the injection of empty liposomes, together with fBSA, was found to be ineffective in inducing an immune response. Liposome-associated protein antigen was found to be a potent stimulator of immunological memory, as demonstrated by the ability of LSM-BSA primed animals to generate a vigorous PFC response upon challenge with the weakly immunogenic fBSA. The injection of congenitally athymic homozygous nude (Nu/Nu) mice with LSM-BSA failed to induce significant antibody formation, whereas the heterozygous (Nu/+) littermates gave a normal PFC response to the same LSM-BSA preparation. Thus, BSA remains a T-cell-dependent antigen, despite its entrapment within liposomes, and T lymphocytes appear to play an obligatory role in providing synergistic interactions for eliciting a BSA-specific PFC response to the LSM-BSA.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C., Davies A. J. Requirement of thymus-dependent lymphocytes for potentiation by adjuvants of antibody formation. Nature. 1971 Oct 1;233(5318):330–332. doi: 10.1038/233330a0. [DOI] [PubMed] [Google Scholar]
- Allison A. G., Gregoriadis G. Liposomes as immunological adjuvants. Nature. 1974 Nov 15;252(5480):252–252. doi: 10.1038/252252a0. [DOI] [PubMed] [Google Scholar]
- Bangham A. D. Properties and uses of lipid vesicles: an overview. Ann N Y Acad Sci. 1978;308:2–7. doi: 10.1111/j.1749-6632.1978.tb22010.x. [DOI] [PubMed] [Google Scholar]
- DIETRICH F. M., WEIGLE W. O. IMMUNOLOGIC UNRESPONSIVENESS TO HETEROLOGOUS SERUM PROTEINS INDUCED IN ADULT MICE AND TRANSFER OF THE UNRESPONSIVE STATE. J Immunol. 1964 Feb;92:167–172. [PubMed] [Google Scholar]
- DRESSER D. W. Specific inhibition of antibody production. II. Paralysis induced in adult mice by small quantities of protein antigen. Immunology. 1962 May;5:378–388. [PMC free article] [PubMed] [Google Scholar]
- Gamble C. N. The role of soluble aggregates in the primary immune response of mice to human gamma globulin. Int Arch Allergy Appl Immunol. 1966;30(5):446–455. doi: 10.1159/000229829. [DOI] [PubMed] [Google Scholar]
- Golub E. S., Mishell R. I., Weigle W. O., Dutton R. W. A modification of the hemolytic plaque assay for use with protein antigens. J Immunol. 1968 Jan;100(1):133–137. [PubMed] [Google Scholar]
- Golub E. S., Weigle W. O. Studies on the induction of immunologic unresponsiveness. 3. Antigen form and mouse strain variation. J Immunol. 1969 Feb;102(2):389–396. [PubMed] [Google Scholar]
- Gregoriadis G., Allison A. C. Entrapment of proteins in liposomes prevents allergic reactions in pre-immunised mice. FEBS Lett. 1974 Sep 1;45(1):71–74. doi: 10.1016/0014-5793(74)80813-6. [DOI] [PubMed] [Google Scholar]
- Gregoriadis G., Buckland R. A. Enzyme-containing liposomes alleviate a model for storage disease. Nature. 1973 Jul 20;244(5412):170–172. doi: 10.1038/244170a0. [DOI] [PubMed] [Google Scholar]
- Gregoriadis G., Ryman B. E. Lysosomal localization of -fructofuranosidase-containing liposomes injected into rats. Biochem J. 1972 Aug;129(1):123–133. doi: 10.1042/bj1290123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath T. D., Edwards D. C., Ryman B. E. The adjuvant properties of liposomes. Biochem Soc Trans. 1976;4(1):129–133. doi: 10.1042/bst0040129. [DOI] [PubMed] [Google Scholar]
- Hoekstra D., Scherphof G. Effect of fetal calf serum and serum protein fractions on the uptake of liposomal phosphatidylcholine by rat hepatocytes in primary monolayer culture. Biochim Biophys Acta. 1979 Feb 20;551(1):109–121. doi: 10.1016/0005-2736(79)90357-2. [DOI] [PubMed] [Google Scholar]
- JOHNSON A. G., GAINES S., LANDY M. Studies on the O antigen of Salmonella typhosa. V. Enhancement of antibody response to protein antigens by the purified lipopolysaccharide. J Exp Med. 1956 Feb 1;103(2):225–246. doi: 10.1084/jem.103.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson H. M., Brenner K., Hall H. E. The use of a wate-soluble carbodiimide as a coupling reagent in the passive hemagglutination test. J Immunol. 1966 Dec;97(6):791–796. [PubMed] [Google Scholar]
- McConahey P. J., Dixon F. J. A method of trace iodination of proteins for immunologic studies. Int Arch Allergy Appl Immunol. 1966;29(2):185–189. doi: 10.1159/000229699. [DOI] [PubMed] [Google Scholar]
- Mishell R. I., Dutton R. W. Immunization of dissociated spleen cell cultures from normal mice. J Exp Med. 1967 Sep 1;126(3):423–442. doi: 10.1084/jem.126.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STAVITSKY A. B., ARQUILLA E. R. Micromethods for the study of proteins and antibodies. III. Procedures and applications of hemagglutination and hemagglutination-inhibition reactions with bis-diazotized benzidine and protein-conjugated red blood cells. J Immunol. 1955 Apr;74(4):306–312. [PubMed] [Google Scholar]
- Sessa G., Weissmann G. Incorporation of lysozyme into liposomes. A model for structure-linked latency. J Biol Chem. 1970 Jul 10;245(13):3295–3301. [PubMed] [Google Scholar]
- Sonoda S., Schlamowitz M. Studies of 125I trace labeling of immunoglobulin G by chloramine-T. Immunochemistry. 1970 Nov;7(11):885–898. doi: 10.1016/0019-2791(70)90051-0. [DOI] [PubMed] [Google Scholar]
- Taylor R. B. Cellular cooperation in the antibody response of mice to two serum albumins: specific function of thymus cells. Transplant Rev. 1969;1:114–149. doi: 10.1111/j.1600-065x.1969.tb00138.x. [DOI] [PubMed] [Google Scholar]
- Tyrrell D. A., Heath T. D., Colley C. M., Ryman B. E. New aspects of liposomes. Biochim Biophys Acta. 1976 Dec 14;457(3-4):259–302. doi: 10.1016/0304-4157(76)90002-2. [DOI] [PubMed] [Google Scholar]
- Uemura K., Nicolotti R. A., Six H. R., Kinsky S. C. Antibody formation in response to liposomal model membranes sensitized with N-substituted phosphatidylethanolamine derivatives. Biochemistry. 1974 Apr 9;13(8):1572–1578. doi: 10.1021/bi00705a003. [DOI] [PubMed] [Google Scholar]
- Yasuda T., Dancey G. F., Kinsky S. C. Immunogenic properties of liposomal model membranes in mice. J Immunol. 1977 Dec;119(6):1863–1867. [PubMed] [Google Scholar]
- Yasuda T., Tadakuma T., Pierce C. W., Kinsky S. C. Primary in vitro immunogenicity of liposomal model membranes in mouse spleen cell cultures. J Immunol. 1979 Oct;123(4):1535–1539. [PubMed] [Google Scholar]
- van Houte A. J., Snippe H., Willers J. M. Characterization of immunogenic properties of haptenated liposomal model membranes in mice. I. Thymus independence of the antigen. Immunology. 1979 Jun;37(2):505–514. [PMC free article] [PubMed] [Google Scholar]
- van Rooijen N., van Nieuwmegen R. Liposomes in immunology: evidence that their adjuvant effect results from surface exposition of the antigens. Cell Immunol. 1980 Feb;49(2):402–407. doi: 10.1016/0008-8749(80)90043-x. [DOI] [PubMed] [Google Scholar]
- van Rooijen N., van Nieuwmegen R. Liposomes in immunology: the immune response against antigen-containing liposomes. Immunol Commun. 1977;6(5):489–498. doi: 10.3109/08820137709094148. [DOI] [PubMed] [Google Scholar]


