Abstract
Objective:
To review basic meniscal anatomy, histology, and biomechanical principles as they apply to surgery and rehabilitation.
Data Sources:
We searched MEDLINE and CINAHL for the years 1960–1999 using the terms meniscus, surgery, rehabilitation, meniscal repair, and arthroscopy.
Data Synthesis:
Injuries to a healthy meniscus are usually produced by a compressive force coupled with transverse-plane tibiofemoral rotation as the knee moves from flexion to extension during rapid cutting or pivoting. The goal of meniscal surgery is to restore a functional meniscus to prevent the development of degenerative osteoarthritis in the involved knee. The goal of rehabilitation is to restore patient function based on individual needs, considering the type of surgical procedure, which meniscus was repaired, the presence of coexisting knee pathology (particularly ligamentous laxity or articular cartilage degeneration), the type of meniscal tear, the patient's age, preoperative knee status (including time between injury and surgery), decreased range of motion or strength, and the patient's athletic expectations and motivations. Progressive weight bearing and joint stress are necessary to enhance the functionality of the meniscal repair; however, excessive shear forces may be disruptive. Prolonged knee immobilization after surgery can result in the rapid development of muscular atrophy and greater delays in functional recovery.
Conclusions/Recommendations:
Accelerated joint mobility and weight-bearing components of rehabilitation protocols represent the confidence placed in innovative surgical fixation methods. After wound healing, an aquatic therapy environment may be ideal during all phases of rehabilitation after meniscal surgery (regardless of the exact procedure), providing the advantages of controlled weight bearing and mobility progressions. Well-designed, controlled, longitudinal outcome studies for patients who have undergone meniscectomy, meniscal repair, or meniscal reconstruction are lacking.
Keywords: biomechanics, knee anatomy, exercise
Knee injuries account for approximately 14% to 16% of all musculoskeletal injuries at the high school level.1,2 The National Athletic Trainers' Association1 ranked knee injury frequency second to the combined frequency of hip, thigh, and leg segment injuries, whereas the Puget Sound Sports Medicine Group ranked knee injuries second only to ankle injuries.2 Stocker et al3 reported that meniscal injuries accounted for 12% of all football knee injuries in a recent high school injury survey. The National Athletic Trainers' Association's high school knee injury survey projected that approximately 9000 knee surgeries are performed annually on high school athletes in the United States.1 Injuries to a healthy meniscus are usually produced by coupled compressive and rotational tibiofemoral joint forces. These forces tend to “pinch” the menisci as they attempt to rapidly conform to the 3-dimensional joint stresses that arise as the compressively loaded knee internally or externally rotates in the transverse plane during sagittal-plane flexion-extension.4 These coupled forces commonly occur during athletic movements that require sudden directional changes such as rapid cutting or pivoting.4
Instantaneous damage to both ligamentous and meniscal structures is more common than isolated injury. The unhappy triad was described by O'Donoghue as an injury to the medial collateral ligament, the anterior cruciate ligament (ACL), and the medial meniscus5,6; however, recent reports suggest that the lateral meniscus is more commonly injured.7–10 Acute ACL disruption associated with sudden transverse-plane rotary forces more commonly damages the lateral meniscus as excessive lateral compartment compression and shear forces stress the posterolateral tibiofemoral articulation.9 Medial meniscus injury is usually associated with repetitious anterior translation in the chronic ACL-deficient knee, disrupting articular surfaces11 and leading to the early onset of osteoarthritis (OA).12
Knee injury management is a concern for most sports medicine health care providers. Our objective is to provide a review of basic anatomic, histologic, and biomechanical principles of the meniscus. This information is then assimilated with current surgical and rehabilitation methods to provide clinicians with a complete overview of the present state of meniscal injury management. The ultimate challenge is to return the athlete to sport with normal or optimal (given the extent of the initial lesion and the surgical method) meniscal function.
ANATOMY
The menisci extend the superior tibial surface, improving its congruency with the femoral condyles.13,14 Both menisci are fibrocartilaginous and wedge shaped in the coronal plane. The medial meniscus is more crescent shaped, and the lateral meniscus is more circular. The superior portions of the menisci are concave, enabling effective articulation with their respective convex femoral condyles, whereas the inferior surfaces are flat to conform to the tibial plateaus. Anterior and posterior meniscal horns attach to the intercondylar eminence of the tibial plateau (Figure 1). The coronary ligaments provide peripheral attachments between the tibial plateau and the perimeter of both menisci. The medial meniscus is also attached to the medial collateral ligament, which limits its mobility. The lateral meniscus is connected to the femur via the anterior (ligament of Humphrey) and posterior (ligament of Wrisberg) meniscofemoral ligaments, which can tension its posterior horn anteriorly and medially with increasing knee flexion.15,16 The transverse ligament provides a connection between the anterior aspects of both menisci. The increased stability provided by the ligamentous attachments prevents the menisci from being extruded out of the joint during compression.17–22
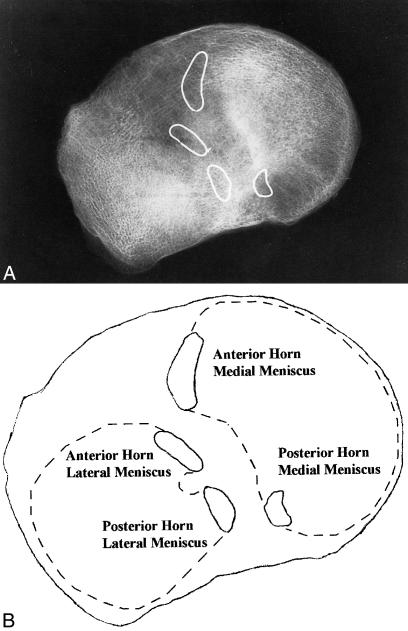
Figure 1.
A, Radiographic anterior and posterior meniscal horn locations. B, Meniscal horn identification in relation to the lateral and medial meniscus (dotted outlines)
Vascular Anatomy
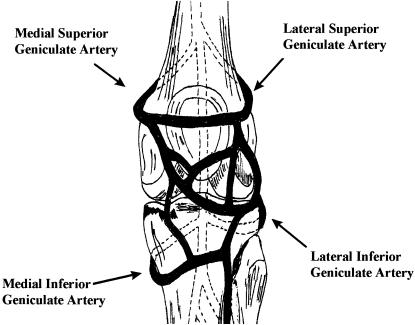
Vascular supply is crucial to meniscal healing. The medial, lateral, and middle geniculate arteries, which branch off the popliteal artery, provide the major vascularization to the inferior and superior aspects of each meniscus (Figure 2).23–27 The middle geniculate artery is a small posterior branch that pierces the oblique popliteal ligament at the posteromedial corner of the tibiofemoral joint. A premeniscal capillary network arising from branches of these arteries originates within the synovial and capsular tissues of the knee along the periphery of the menisci. Only 10% to 30% of the peripheral medial meniscus border and 10% to 25% of the lateral meniscus border receive direct blood supply.23,24 Endoligamentous vessels from the anterior and posterior horns travel a short distance into the substance of the menisci and form terminal loops, providing another direct route for nourishment.23 The remaining portion of each meniscus (65% to 75%) receives nourishment only from the synovial fluid via diffusion.28,29
Figure 2.
Confluence of geniculate arteries (anterior view).
Neuroanatomy
The knee joint is innervated by the posterior articular branch of the posterior tibial nerve and the terminal branches of the obturator and femoral nerves. Nerve fibers penetrate the joint capsule, along with the vascular supply, and service the substance of the menisci. Ruffini, Pacinian, and Golgi tendon mechanoreceptors have been identified in the knee joint capsule and in the peripheral menisci.30–36 Type I (Ruffini) mechanoreceptors are low threshold and slowly adapting to changes in static joint position and pressure. Type II (Pacinian) mechanoreceptors are low threshold and fast adapting to tension changes, signaling joint acceleration.30–36 Type III (Golgi) mechanoreceptors signal when the knee joint approaches the terminal range of motion (ROM) and are associated with neuromuscular inhibition. Concentrations of meniscal mechanoreceptors (especially Pacinian mechanoreceptors) are greatest in the meniscal horns, leading researchers to study their contributions to proprioception.30–36
BIOMECHANICS
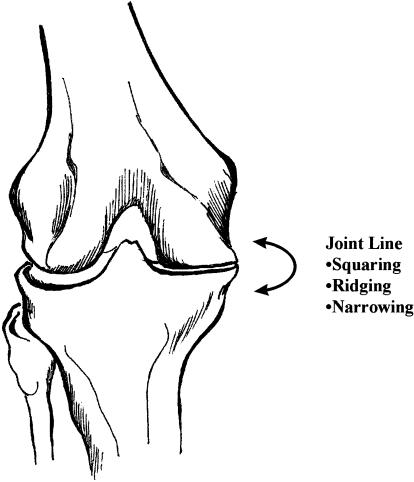
The major meniscal functions are to distribute stress across the knee during weight bearing,37,38 provide shock absorption,37,39,40 serve as secondary joint stabilizers,41–44 provide articular cartilage nutrition and lubrication, facilitate joint gliding, prevent hyperextension, and protect the joint margins.17,18 Circumferential meniscal stress measurements have shown that 45% to 70% of the weight-bearing load is transmitted through the menisci when the peripheral margins are intact.17,18,21,29,39 The biomechanical adaptations of the meniscectomized knee show a doubling of joint contact stress in conjunction with a 50% to 70% reduction in contact area.45 A 10% reduction in meniscal contact area secondary to partial meniscectomy reportedly produces a 65% increase in peak joint-contact stresses,45 leading to the early development of OA.46,47 Radiographically, the Fairbank sign indicates joint space narrowing due to osteophyte formation from increased peak joint stresses (Figure 3).46
Figure 3.
Medial joint line degeneration: the Fairbank sign.
During knee flexion, the femoral condyles glide posteriorly on the tibial plateau in conjunction with tibial internal rotation. The lateral meniscus undergoes twice the anteroposterior translation of the medial meniscus during knee flexion (11.2 mm versus 5.1 mm).22 This translation prevents the femur from contacting the posterior margin of the tibial plateau. The medial condyle rolling-to-translation ratio is 1:1, whereas the lateral condyle ratio is 1:4.19 The lateral meniscus can better accommodate this mobility by translating with the femoral condyles and is thereby less susceptible to injury than the medial meniscus.19 The congruity of the tibiofemoral articulation is maintained throughout complete knee ROM via healthy, mobile menisci.13,48
HISTOLOGY AND STRUCTURE
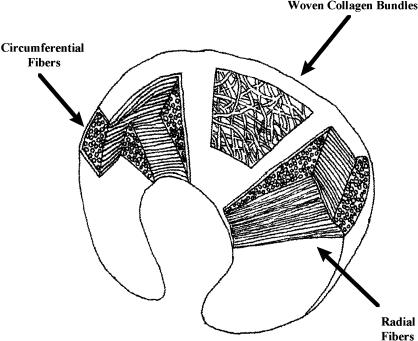
The microstructural characteristics of the menisci dictate their mechanical properties. The menisci are composed of 70% water and 30% organic matter. Collagen constitutes 75% of the organic matter, while roughly 8% to 13% of the remaining dry matter consists of noncollagenous proteins.39,49–52 Type I collagen fibers provide the primary meniscal structural scaffolding; this predominance of type I collagen is one of the major differences between the menisci and hyaline, or articular, cartilage, which is composed of predominantly type II collagen.48,53 The cellular meniscal components also include fibrochondrocytes interspersed within the extracellular matrix. Fibrochondrocytes display the properties of both fibroblasts and chondrocytes, synthesizing and maintaining the extracellular matrix, especially the collagen.48 Three collagen fiber layers are specifically arranged to convert compressive loads into circumferential or “hoop” stresses (Figure 4). In the superficial layer, the fibers travel radially, serving as “ties” that resist shearing or splitting. In the middle layer, the fibers run parallel or circumferentially to resist hoop stress during weight bearing. Lastly, there is a deep layer of collagen bundles that are aligned parallel to the periphery.54 The remainder of the extracellular matrix is composed of proteoglycans. The glycosaminoglycans (GAGs), or chains of proteoglycan aggregates, make up only 1% of the wet weight of the meniscus but contribute most to its material properties, such as tissue hydration, compressive stiffness, and elasticity.28,55 The size of these proteoglycan macromolecules in combination with water-retention and electrostatic-repulsion properties is what gives the menisci their compressive stiffness.10,55 Meniscal shock absorption is time dependent due to the exudation of water out of the extracellular matrix. The exudation of water from the GAG substances provides not only compressive stiffness but also joint lubrication as water is forced into the joint space. The highest GAG concentrations are found in the meniscal horns and the inner half of the menisci, coinciding with the primary weight-bearing areas.56 Meniscal tissue also displays the time-dependent viscoelastic property of “creep,” deforming over time when loading occurs with greater frequency or duration. The proteoglycans add little to meniscal tensile properties. Rather, elastin, which constitutes less than 0.06% of meniscal tissue, is believed to aid in the recovery of shape after load deformation.55
Figure 4.
Meniscal collagen configuration.
CLINICAL EXAMINATION
The need for surgery after meniscal injury is largely determined from the data obtained during the initial physical examination in conjunction with other diagnostic tests. A comprehensive examination should include a thorough injury history, regional palpation, and select special tests.4 Athletes with meniscal tears commonly describe feeling a pop while performing a sudden running directional change, such as rapid cutting or pivoting, with or without contact with another player.4 Johnson et al57 reported that statistical methods applied to medical historical data were 85% to 98% accurate for predicting the presence of a meniscal tear, depending on whether 30 or 142 predictor questions were used. Knee joint-line tenderness and effusion are also associated with meniscal lesions. Knee joint-line palpation may produce equivocal results, with medial joint-line specificity of 34.5% and sensitivity of 44.9% for predicting medial meniscal tears and lateral joint-line specificity of 49.1% and sensitivity of 57.6% for predicting lateral meniscal tears in subjects with acute ACL injuries.58 Among subjects with a nonimpaired ACL, knee joint-line tenderness is more accurate, with a 77% clinical accuracy for meniscal tear identification.59,60 Dye et al,61 using conscious neurosensory mapping of intra-articular knee joint structures, reported poor pain localization at the cruciate ligaments and the menisci. Given what we know regarding mechanoreceptor distributions and tissue proximity, peripheral meniscal tears are more easily identifiable via palpation. After isolated meniscal tears, results of the Lachman and anterior drawer tests are negative for ACL involvement, as are the results of the apprehension test for patellofemoral instability. The results of the McMurray rotation test and the medial-lateral grind test are 58%60 to 83%62 and 68%60 accurate, respectively, for meniscal tear identification. Athletes with a meniscal tear may also have difficulty performing active, involved-side weight-bearing movements, such as squats or lunges. Conventional radiographs can eliminate the possibility of a fracture, osteochondral injury, or intra-articular loose body. Knee joint arthrography is an invasive method of meniscal lesion identification with poor diagnostic accuracy rates, depending greatly upon the skills and experience of the examiner.63 Magnetic resonance imaging, with accuracy rates of 90% to 98% for the identification of meniscal tears, has become the radiographic procedure of choice; however, it is more costly than arthrography or conventional radiographic evaluation.64
MENISCECTOMY VERSUS REPAIR
The first reported surgical human meniscus repair occurred in 1885 and was described as tedious.65 Because meniscal repair was considered technically challenging and the meniscus was viewed as a vestigial structure, total meniscectomy became the preferred operation. The early onset of OA was the long-term result of total meniscectomies, prompting the exploration of other surgical options. The partial meniscectomy has since replaced the total meniscectomy as the surgery of choice, along with other options such as repairs and transplantations. Before selecting a particular surgical technique, the surgeon considers the patient's age, health, lifestyle, and willingness to undergo major surgery and the location and type of meniscal tear.66–70 Patients should be educated about the pros and cons of meniscal resection or repair and the extent of their rehabilitation obligation. Older or more sedentary patients are generally more effectively treated with a conventional partial meniscectomy. Patients should also be informed as to the likelihood of surgical success based on which meniscus was injured and the type of tear that occurred. Gillquist and Oretorp71 reported that patients who underwent partial lateral meniscectomy did less well than those who underwent partial medial meniscectomy. Northmore-Ball and Dandy72 reported a slightly greater frequency of excellent clinical results after partial medial meniscectomy than after partial lateral meniscectomy. Patients older than 50 years are considered ideal candidates for partial meniscectomy because they are likely to have degenerative meniscal tissue associated with OA. Degenerative meniscal tears display poor repair potential due to the insufficient tissue integrity of both the lesion site and the adjacent meniscal tissue. Some authors have also recommended partial meniscectomy as the surgical treatment of choice for patients older than 30 years.73
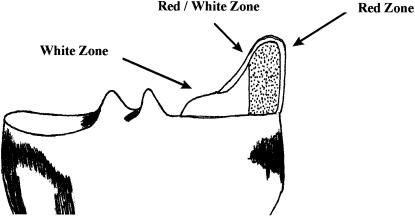
The major determinants of whether a meniscal tear is amenable to surgical repair are the location of the tear, the type of lesion, and its related vascular supply. Three zones determine the healing prognosis for meniscal lesions: red-red, red-white, and white-white (Figure 5). The red-red zone is fully vascular and therefore has an excellent healing prognosis. The red-white zone is at the border of vascular supply and has a generally good healing prognosis. The white-white zone is relatively avascular and has a poor prognosis for healing.74 Arnoczsky and Warren75 and Weiss et al76 have substantiated these findings in studying the contributions of the peripheral microvascular supply to the menisci. DeHaven and Stone77 have suggested that meniscal repairs be performed within 3 mm of the vascular periphery.
Figure 5.
Meniscal healing zones.
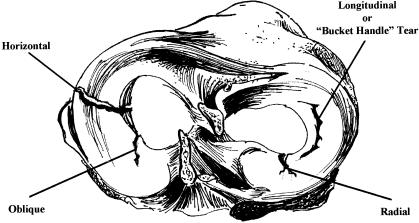
Longitudinal meniscal tears occur parallel to the direction of the circumferential fibers. A bucket-handle tear is a variant of a longitudinal tear in that the circumferential fibers are also disrupted as the tear travels from the innermost aspect of the meniscus toward the periphery (Figure 6). The vertical longitudinal meniscal tear is considered ideal for repair because of minimal circumferential fiber disruption.74 In contrast, the bucket-handle tear is less amenable to repair, as stray circumferential fibers may interfere with healing.74 Flap and radial meniscal tears also disrupt these circumferential collagen fibers and are more amenable to debridement than repair. Degenerative meniscal tears involve multiple tissue-cleavage planes, delamination, and calcified cyst formation associated with related OA signs, such as osteophytes and articular cartilage damage, and almost always warrant debridement rather than repair. Henning78 reported better results when meniscal repairs were performed within 8 weeks of initial injury and an increased likelihood of OA the longer the time period from the initial injury.7 Good results, however, have been reported 8 weeks or more after initial injury, provided the location and meniscal lesion type met the repair criteria.69
Figure 6.
Types of meniscal tears.
Meniscal repair procedures are divided into 2 major types: open and arthroscopically assisted. The open procedure is less common because of the greater tissue trauma associated with larger surgical incisions, although good results have been reported 10 years after open medial meniscal repair.78 The “all-inside” technique is an arthroscopic procedure with the benefits of smaller incisions and a reduced risk of neurovascular injury, particularly when peripheral tears within the meniscal red zone are repaired.79 A variation of the all-inside technique is the use of permanent80 or biodegradable81 transmeniscal sutures requiring only 1 surgical incision. “Inside-out”82,83 and “outside-in”84 arthroscopically assisted meniscal repair techniques (named by the origin of suture delivery) have also been reported; however, they may place adjacent neurovascular structures at a greater risk of injury.23,68 Neurovascular complications generally involve lower extremity weakness and sensory loss; therefore, regular postoperative monitoring of neurovascular integrity should be performed.
MENISCAL REPAIR FACILITATORS
The poor healing of white-white zone meniscal lesions has prompted researchers to explore options to enhance healing. Presently, 3 methods are commonly used to enhance healing after meniscal repair: fibrin clot injection, vascular access channel creation, and synovial abrasion.37,50,77,85
Fibrin Clot
A fibrin clot can be injected into the meniscal lesion to promote healing through hematoma chemotactic factors.50 Arnoczky et al50 injected a fibrin clot matrix into the meniscal defects of dog knees and reported healed tissue resembling normal meniscus 6 months later. Hashimoto et al,86 using similar methods and mechanical testing, reported that the healed meniscus was less able to resist deformation than normal tissue. The results of both studies suggested that adjacent meniscal fibrochondrocytes or surrounding synovial fluid provided the primary biological stimulus for tissue repair. Henning et al87 used a fascial sheath to cover fibrin clots placed in human meniscal defects and observed that 32% of the defects healed completely and 52% healed incompletely by 6 months after surgery.
Vascular Access Channels
Vascular access channels (trephination) are tunnels created from vascular portions of the peripheral meniscus (red zone) to the more central avascular area (white zone). Theoretically, trephination enables fibrovascular scar proliferation in the damaged meniscal section. Fox et al,88 using a patient survey and clinical examination, reported good to excellent results for 90% of patients with incomplete meniscal tears treated with trephination. Using a goat model, Zhang et al89 reported at least partial healing by 25 weeks after combined trephination and meniscal defect suturing of longitudinal tears in the avascular area.
Synovial Abrasion
Abrasion of the synovium with a surgical rasping device activates chemotactic factors that stimulate meniscal healing. Excessive synovectomy during meniscal debridement has been shown to prevent meniscal regeneration.90 Synovial cell migration to the meniscal defect may enhance healing, with less effective healing occurring when the distance between the abraded synovium and the defect is increased.91 Surgeons generally abrade the margins and superficial layer of the meniscal tear to further promote healing.83,92–94
LASERS
Lasers are more commonly being used in the ablation, or destruction, of damaged meniscal material during arthroscopic meniscectomies. The proposed mechanisms of ablation are photothermal, photochemical, and photomechanical, with each mechanism having different biological effects.95 The photothermal effect of long-pulse, continuous-wave lasers can vaporize tissue. Photochemical effects occur from the dissociation of molecular tissue bonds, also called photoablative decomposition. Photomechanical effects occur from tissue exposure to short laser pulses, which stress the tissue beyond its mechanical strength. An example of an excessive photomechanical effect is the thermoelastic expansion caused by bubble formation within meniscal tissue after laser intervention.96 Tissue cavitation in the presence of tensile stresses can lead to further degenerative changes.96 Forman et al97 studied the use of a laser to promote in vitro healing in human menisci that had also received a fibrin clot and reported that the laser helped prevent clot matrix displacement, allowing more time for fibrochondrocyte absorption into the meniscal defect.
MENISCAL RECONSTRUCTION
A meniscal allograft is donated from a cadaver and transplanted into an injured knee. There are 4 types of meniscal allograft preparations: fresh, deep frozen, cryopreserved and freeze dried. Each preparation has pros and cons. Although the fresh meniscal allograft contains functioning fibrochondrocytes, the window of opportunity for transplantation is only a few days. Also, the threat of human immunodeficiency virus (HIV) infection is a possibility when fresh tissue is used (approximately 1 person in 22 629 has a positive test result for the virus).98 Both the deep-frozen and cryopreservation methods eliminate the threat of HIV infection, but they also reduce the amount of viable meniscal tissue and alter its biomechanical properties. Both alteration of the tissue biomechanical properties and improper allograft sizing can lead to postoperative failure. Velteri et al,99 using a dog model, reported that cellular components in both cryopreserved and deep-frozen meniscal allografts were eventually fully replaced.
Fresh Allografts
Garrett and Stevensen100 reported that fresh human allograft fixation by peripheral suturing to surrounding fibrous tissue resulted in no evidence of meniscal degeneration at 44 months after implantation; however, radiographic assessment revealed joint space narrowing on the side of the transplant. Biopsies revealed that transplanted menisci retained their original size and shape, suggesting that the chondrocytes continued to produce glycoproteins.100 Jackson et al101 reported normal vascular distributions in meniscal allografts up to 6 months after transplantation but noted decreased water and proteoglycan content and overall cellularity. Keating,102 using a goat model, reported an inflammatory infiltrate at 3 months after transplantation and articular destruction at 7 months, suggesting failure linked to immune responses. Urban et al103 emphasized proper meniscal horn placement when attempting to restore functionality to a transplanted meniscus, regardless of the preparation method. The long-term effectiveness of fresh meniscal transplants will not be fully realized until a long-term longitudinal study is completed.
Deep-Frozen Allografts
Meniscal transplantation using frozen donor tissue is probably the simplest and least expensive method; however, the process is known to destroy donor fibrochondrocytes and partially shrink the graft tissue.104,105
Cryopreserved Allografts
During cryopreservation (Cryolife, Marietta, GA), the graft tissue is frozen in glycerol, thereby preserving cell membrane integrity and donor fibrochondrocyte viability.105 The graft is gradually thawed before transplantation. Cell viability may vary depending on graft size, preservation medium, and freezing or thawing rate.106 Cryopreservation allows for longer graft storage durations, more time for serologic testing, and more precise sizing, but it is expensive and technically challenging. Arnoczsky et al107 used a dog model to study the histologic properties of cryopreserved meniscal tissue and reported that the number of metabolically active cells after transplantation decreased and cellularity and peripheral vascularity increased by 3 months after surgery. Fissuring of the adjacent tibial condyle hyaline cartilage was also evident at 6 months after surgery.107 Speculation continues over the long-term effects of cryopreserved meniscal allografts, and controlled longitudinal studies are needed.
Freeze-Dried Allografts
Freeze-dried grafts are frozen after vacuum dehydration. Before transplantation, the graft is thawed and rehydrated, tending to increase graft fragility. After transplantation, the graft serves as a scaffold for the ingrowth of host fibrochondrocytes. Freeze-dried meniscal allografts provide ease in handling and prolonged storage life at room temperature.108 However, the results from freeze-dried meniscal allograft transplantation have not been encouraging because of histologic alterations, such as cartilaginous degeneration and synovitis.108 Since the freeze-drying process does not destroy HIV in blood products, disease transmission is also possible.
MENISCAL REGENERATION
Collagen scaffolds from exogenous sources such as man-made polymers may eventually provide the properties necessary for fibrochondrocyte ingrowth to facilitate meniscal regeneration in humans. Using “re-look” arthroscopy to evaluate 8 patients a minimum of 24 months after collagen meniscus implantation, Rodkey et al109 observed tissue regeneration and joint surface preservation. A collagen scaffold with appropriate pore size must enhance fibrochondrocyte proliferation, avoid immunologic responses, provide stability, prevent the onset of OA, and subsequently degenerate.109 Histologic studies have shown variable results, and further clinical trials are needed to evaluate the ability of scaffolds to protect the articular cartilage of the human femoral condyle.
REHABILITATION
Rehabilitation after partial meniscectomy can generally progress as tolerated with no substantial contraindications or limitations. The main goals are to control the pain and inflammation associated with surgery, maintain ROM and general conditioning, restore or maintain isolated muscle function, and optimize integrated lower extremity neuromuscular coordination. Immediate progressive ROM and neuromuscular reeducation and strengthening are warranted. A concurrent goal is the control of effusion, pain, and inflammation with cryotherapy and the use of nonsteroidal, anti-inflammatory medication. Clinicians should be vigilant in assessing changes in patellofemoral, patellar tendon, and tibiofemoral joint line irritability via regular palpation, particularly after advancing an existing exercise program.
In a prospective, randomized study, Jokl et al110 found that a well-planned, unsupervised rehabilitation program enables patients undergoing arthroscopic knee surgery to return to sports within the same time frame as patients who receive supervised physical therapy. In contrast, Moffet et al,111 in a randomized, controlled study, showed that patients who received supervised rehabilitation had more rapid recovery of the quadriceps femoris muscle than did patients in an unsupervised control group; the authors concluded that early and intensive rehabilitation was vital to successful functional outcomes after partial meniscectomy.111 Matthews and St-Pierre112 reported that patients require 4 to 6 weeks for the quadriceps femoris and 4 weeks for the hamstrings to return to preoperative isokinetic strength levels after partial meniscectomy. St-Pierre113 suggested that preoperative knee extensor-flexor strength deficits increase the need for supervised rehabilitation. Clinicians should be familiar with the specifics of the aforementioned surgical procedures to safely advance and, if necessary, modify rehabilitation program progressions. Most progressions focus on 2 major areas: immobilization and weight-bearing status.
Immobilization
Immobilization after meniscal repair has been recommended at or near full extension (10° to 20° of flexion) to better approximate longitudinal tears.83,84,93,94 Zhang et al,114 using a rabbit model, reported that immobilization has a greater influence than suture use on the healing rate of meniscal tears. Studies in dogs have substantiated this finding, with excellent healing rates using only immobilization.115 Eriksson and Haggmart,116 however, reported that atrophy during immobilization can decrease the human quadriceps femoris mass by 40% by 5 weeks after major knee surgery. Traditionally, a brief period (4 to 6 weeks) of decreased mobility after meniscal repair is recommended; however, the authors of more recent reports recommend decreasing the immobilization period, even in the absence of sutures.92,93,117 Those authors advocating early ROM have not reported deleterious effects on meniscal repairs118 and have suggested that this approach improves articular cartilage health.119
Weight Bearing
The findings of many studies support weight-bearing limitations during the initial 4 to 8 weeks after meniscal repair.77,83,92–94 In theory, weight bearing alone should not disrupt healing meniscal tissue, because the hoop stresses are primarily absorbed at the periphery of the meniscus. More recent reports have recommended earlier weight bearing to promote the restoration of a functional meniscus via the clinical application of Wolff's law.120 Weight bearing in conjunction with tibiofemoral rotation during knee flexion, however, could produce shear forces capable of disrupting healing meniscal tissue, particularly if the fixation strength is inadequate.121
ACCELERATED REHABILITATION
DeHaven and Bronstein122 described a meniscal repair rehabilitation protocol of an initial 2 weeks of maximum protection (immobilization at 0° of flexion, toe-touch weight bearing), 4 weeks of protected ROM (30° to 70° of flexion), and controlled knee extensor-flexor strengthening and full weight bearing after 6 weeks. Stationary cycling and moderate-intensity running were allowed between 3 and 6 months after surgery, and full return to activity was allowed at 7 months after surgery. Although the success rates for protocols similar to the protocol suggested by DeHaven and Bronstein122 were consistently high (75% to 95%), more recent reports suggest that earlier application of controlled stress to the repaired meniscus may enhance its functionality.68,71,83,92,94,117,123 Barber124 reported no differences in healing rates between patients who followed a standard rehabilitation program (protective) and patients who followed a program that permitted immediate weight bearing, unbraced motion, unlimited exercise performance, and an early return to pivoting-type sports movements. Barber124 emphasized avoiding standard “cookbook” protocols and encouraged individualized programs based on the type of surgical procedure, which meniscus was repaired, the presence of coexisting knee pathology (ie, ligamentous laxity or OA), meniscal tear type, the patient's age, preoperative knee status (including the time between injury and surgery), loss of ROM and strength, and the patient's athletic expectations and motivations.
Accelerated meniscal repair rehabilitation programs that permit full knee ROM and full weight bearing are becoming more common, with return to full activity as early as 10 weeks after surgery.110,120,124 Although studies have documented short-term successes, controlled longitudinal studies are needed to determine long-term outcomes. Barber and Click,125 in evaluating 63 patients with 65 meniscal repairs at a minimum of 2 years after surgery, reported successful healing in 92% of patients (53 of 58) who had an ACL reconstruction during the same surgery but in only 67% of patients (2 of 3) who were not treated for ACL deficiency. The authors suggested that patients who undergo combined ACL reconstruction and meniscal repair can safely follow the same accelerated protocol as patients who only undergo meniscal repair.125 Shelbourne et al120 reported that patients undergoing combined ACL reconstruction and meniscal repair who perform immediate postoperative ROM and weight bearing as tolerated have comparable clinical results to patients who follow a more restrictive rehabilitation protocol. Mintzer et al126 assessed the return-to-activity level in 29 patients who were 17 years of age or younger at the time of meniscal repair. At an average of 5 years after surgery, 100% of the patients had excellent clinical results (full ROM, no effusion, no joint-line tenderness, no joint locking), and 85% had returned to sports with cutting or pivoting components.126 To date, the follow-up period for accelerated rehabilitation after meniscal repair is too brief to declare its superiority over more traditional progressions.126
Given the concerns over attempting to provide an ideal weight-bearing and knee ROM progression, aquatic therapy may provide an excellent rehabilitation method following meniscal repair (after surgical wound healing). Tovin et al127 and Kuhne and Zirkel128 reported on the efficacy of aquatic therapy for patients after ACL reconstruction. By varying water depths and using flotation devices, weighted devices (vests, belts), and resistive devices (tubing, vented fins, etc), patients can safely progress from knee ROM and neuromuscular recovery activities to more aggressive muscle strength and endurance challenges and sport-specific functional movement patterns (eg, hopping and jumping tasks). An aquatic environment is also a relatively safe exercise location for patients requiring long-term restriction from the compressive forces associated with dry-land running and jumping.
SUMMARY
A comprehensive knowledge of the meniscus is necessary to effectively manage the rehabilitation of patients after meniscal injury and surgery. The unique biomechanical and histologic properties of the meniscus must be preserved to maintain knee health. Clinicians who rehabilitate patients with meniscal injuries should be familiar with normal meniscal anatomy, physiology, and biomechanics as they apply to surgery and rehabilitation. Historically, the lack of appreciation for normal meniscal function resulted in total surgical removal, prompting a proliferation of knee joint OA. Innovations in surgical techniques have led to increased meniscal tissue preservation to minimize the long-term sequelae after injury. In addition to the current interest in repair and transplantation, interest is growing in the use of exogenous materials to facilitate meniscal regeneration and the use of tissue growth factors and even gene therapy to restore a functional meniscus. Surgical innovations are progressing more rapidly than the acquisition and interpretation of long-term surgical and rehabilitation outcome data. Well-designed, longitudinal studies of surgical and rehabilitation outcomes are imperative to determine the actual efficacy of any of these procedures with regard to patient function and satisfaction.
REFERENCES
- 1.National high school injury survey. Natl Athl Train Assoc News. 1996 Apr;:17–23. [Google Scholar]
- 2.Rice SG. Risks of injury during sports participation. In: Sullivan JA, Anderson SJ, editors. Care of the Young Athlete. Rosemont, IL: American Academy of Orthopaedic Surgeons and the American Academy of Pediatrics; 2000. pp. 9–18. [Google Scholar]
- 3.Stocker B, Nyland J, Caborn D, Sternes R, Ray JM. Results of Kentucky high school football knee injury survey. J Ky Med Assoc. 1997;95:458–464. [PubMed] [Google Scholar]
- 4.Wheatley WB, Krome J, Martin DF. Rehabilitation programmes following arthroscopic meniscectomy in athletes. Sports Med. 1996;21:447–456. doi: 10.2165/00007256-199621060-00006. [DOI] [PubMed] [Google Scholar]
- 5.O'Donoghue DH. Surgical treatment of fresh injuries to the major ligaments of the knee [classical article] 1950. Clin Orthop. 1991;271:3–8. [PubMed] [Google Scholar]
- 6.O'Donoghue D. An analysis of end results of surgical treatment of major injuries to the ligaments of the knee. J Bone Joint Surg Am. 1955;37:19–22. [PubMed] [Google Scholar]
- 7.Barber F. Accelerated rehabilitation for meniscus repairs. Arthroscopy. 1994;10:206–210. doi: 10.1016/s0749-8063(05)80095-7. [DOI] [PubMed] [Google Scholar]
- 8.Shelbourne KD, Nitz PA. The O'Donoghue triad revisited: combined knee injuries involving anterior cruciate and medial collateral ligament tears. Am J Sports Med. 1991;19:474–477. doi: 10.1177/036354659101900509. [DOI] [PubMed] [Google Scholar]
- 9.Barber F. Snow skiing combined anterior cruciate ligament/medial collateral ligament disruptions. Arthroscopy. 1994;10:85–89. doi: 10.1016/s0749-8063(05)80297-x. [DOI] [PubMed] [Google Scholar]
- 10.Duncan JB, Hunter R, Purnell M, Freeman J. Meniscal injuries associated with acute anterior cruciate ligament tears in alpine skiers. Am J Sports Med. 1995;23:170–172. doi: 10.1177/036354659502300208. [DOI] [PubMed] [Google Scholar]
- 11.Mow V, Lai W, Hou J. A triphasic theory for the swelling properties of hydrated charged soft biological tissues. Appl Mech Rev. 1990;43:134–141. [Google Scholar]
- 12.Arnold JA, Coker TP, Heaton LM, Park JP, Harris WD. Natural history of anterior cruciate ligament tears. Am J Sports Med. 1979;7:305–313. doi: 10.1177/036354657900700601. [DOI] [PubMed] [Google Scholar]
- 13.Arnoczky S. Gross and vascular anatomy of the meniscus and its role in meniscal healing. In: Mow VC, Arnoczky S, Jackson D, editors. Knee Meniscus: Basic and Clinical Foundations. New York, NY: Raven Press; 1992. pp. 1–14. [Google Scholar]
- 14.Fu FH, Thompson W. Mow VC, Arnoczky S, Jackson D. Knee Meniscus: Basic and Clinical Foundations. New York, NY: Raven Press; 1992. Motion of the meniscus during knee flexion; pp. 75–90. [Google Scholar]
- 15.Yamamoto M, Hirohata K. Anatomical study on the menisco-femoral ligaments of the knee. Kobe J Med Sci. 1991;37:209–226. [PubMed] [Google Scholar]
- 16.Heller L, Langman J. The menisco-femoral ligaments of the human knee. J Bone Joint Surg Br. 1964;46:307–313. [PubMed] [Google Scholar]
- 17.Seedholm B. Transmission of the load in the knee with special reference to the role of the meniscus: part I. Eng Med. 1979;8:207–221. [Google Scholar]
- 18.Seedholm B, Hargeaves D. Transmission of the load in the knee with special reference to the role of the meniscus: part II. Eng Med. 1979;8:221–228. [Google Scholar]
- 19.Shapeero LB, Dye SF, Lipton MJ, Gould RG, Galvin EG, Genant HK. Functional dynamics of the knee joint by ultrafast, cine CT. Invest Radiol. 1988;23:118–123. doi: 10.1097/00004424-198802000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Shoemaker S, Markolf KL. The role of the meniscus in the anterior-posterior stability of the loaded anterior cruciate deficient knee: effects of partial versus total excision. J Bone Joint Surg Am. 1986;68:71–79. [PubMed] [Google Scholar]
- 21.Shrive NB, O'Connor JJ, Goodfellow JW. Load-bearing in the knee joint. Clin Orthop. 1978;131:279–287. [PubMed] [Google Scholar]
- 22.Thompson WD, Thaete FL, Fu FH, Dye SF. Tibial meniscal dynamics using three-dimensional reconstruction of magnetic resonance images. Am J Sports Med. 1991;19:210–215. doi: 10.1177/036354659101900302. [DOI] [PubMed] [Google Scholar]
- 23.Arnoczky SP, Warren RF. Microvasculature of the human meniscus. Am J Sports Med. 1982;10:90–95. doi: 10.1177/036354658201000205. [DOI] [PubMed] [Google Scholar]
- 24.Danzig L, Resnik D, Gonsalves M, Akeson WH. Blood supply to the normal and abnormal meniscus of the human knee. Clin Orthop. 1983;172:271–276. [PubMed] [Google Scholar]
- 25.Davies D, Edwards D. The vascular and nerve supply of the human meniscus. Am R Coll Surg Engl. 1948;2:142–156. [PMC free article] [PubMed] [Google Scholar]
- 26.Day B, Mackenzie WG, Shim SS, Leung G. The vascular and nerve supply of the human meniscus. Arthroscopy. 1985;1:58–62. doi: 10.1016/s0749-8063(85)80080-3. [DOI] [PubMed] [Google Scholar]
- 27.Scapinelli R. Studies on the vasculature of the human knee joint. Acta Anat. 1968;70:305–331. doi: 10.1159/000143133. [DOI] [PubMed] [Google Scholar]
- 28.Meyers E, Zhu W, Mow V. Viscoelastic properties of articular cartilage and meniscus. In: Nimni M, editor. Collagen: Chemistry, Biology and Biotechnology. Boca Raton, FL: CRC; 1988. [Google Scholar]
- 29.Mow V, Fithian D, Kelly M. Fundamentals of articular cartilage and meniscus biomechanics. In: Ewing JW, editor. Articular Cartilage and Knee Joint Function: Basic Science and Arthroscopy. New York, NY: Raven Press; 1989. pp. 1–18. [Google Scholar]
- 30.Schutte MJ, Dabezius EJ, Zimny ML, Happe LT. Neural anatomy of the human anterior cruciate ligament. J Bone Joint Surg Am. 1987;69:243–247. [PubMed] [Google Scholar]
- 31.Gardner E. The innervation of the knee joint. Anat Rec. 1948;101:109–130. doi: 10.1002/ar.1091010111. [DOI] [PubMed] [Google Scholar]
- 32.Kennedy JC, Alexander IJ, Hayes KC. Nerve supply of the human knee and its functional importance. Am J Sports Med. 1982;10:329–335. doi: 10.1177/036354658201000601. [DOI] [PubMed] [Google Scholar]
- 33.Assimakopoulos AP, Katonis PG, Agapitos MV, Exarchou EI. The innervation of the human meniscus. Clin Orthop. 1992;275:232–236. [PubMed] [Google Scholar]
- 34.O'Connor BL. The histological structure of dog knee menisci with comments on its possible significance. Am J Anat. 1976;147:407–417. doi: 10.1002/aja.1001470402. [DOI] [PubMed] [Google Scholar]
- 35.O'Connor BL, McConnaughey JS. The structure and innervation of cat knee menisci and their relation to a “sensory hypothesis” of meniscal function. Am J Anat. 1978;153:431–442. doi: 10.1002/aja.1001530306. [DOI] [PubMed] [Google Scholar]
- 36.Zimny ML. Mechanoreceptors in articular tissues. Am J Anat. 1988;182:16–32. doi: 10.1002/aja.1001820103. [DOI] [PubMed] [Google Scholar]
- 37.Arnoczky S, Adams M, Mow V. The meniscus. In: Buckwalter J, Woo S, editors. The Injury and Repair of Musculoskeletal Soft Tissue. Park Ridge, IL: American Academy of Orthopaedic Surgeons; 1988. pp. 487–537. [Google Scholar]
- 38.Levy IM, Torzilli PA, Warren RF. The effect of medial meniscectomy on anterior-posterior motion of the knee. J Bone Joint Surg Am. 1982;64:883–888. [PubMed] [Google Scholar]
- 39.Fithian DC, Kelly MA, Mow VC. Material properties and structure-function relationships in the menisci. Clin Orthop. 1990;252:19–31. [PubMed] [Google Scholar]
- 40.Voloshin AS, Wosk J. Shock absorption of meniscectomized and painful knees: a comparative in vivo study. J Biomed Eng. 1983;5:157–161. doi: 10.1016/0141-5425(83)90036-5. [DOI] [PubMed] [Google Scholar]
- 41.Levy IM, Torzilli PA, Gould JD, Warren RF. The effect of lateral meniscectomy on motion of the knee. J Bone Joint Surg Am. 1989;71:401–406. [PubMed] [Google Scholar]
- 42.Radin EL, de Lamotte F, Maquet P. Role of menisci in distribution of stress in the knee. Clin Orthop. 1984;185:290–294. [PubMed] [Google Scholar]
- 43.Fukubayashi T, Torzilli PA, Sherman MF, Warren RF. An in vitro biomechanical evaluation of anterior-posterior motion of the knee: tibial displacement, rotation, and torque. J Bone Joint Surg Am. 1982;64:258–264. [PubMed] [Google Scholar]
- 44.Krause WR, Pope MH, Johnson RJ. Mechanical changes in the knee after meniscectomy. J Bone Joint Surg Am. 1976;58:599–604. [PubMed] [Google Scholar]
- 45.Baratz ME, Fu FH, Mengato R. Meniscal tears: the effect of meniscectomy and of repair on intraarticular contact areas and stress in the human knee. A preliminary report. Am J Sports Med. 1986;14:270–275. doi: 10.1177/036354658601400405. [DOI] [PubMed] [Google Scholar]
- 46.Fairbank T. Knee joint changes after meniscectomy. J Bone Joint Surg Br. 1948;30:664–670. [PubMed] [Google Scholar]
- 47.Jones RE, Smith EC, Reisch JS. Effects of medial meniscectomy in patients older than forty years. J Bone Joint Surg Am. 1978;60:783–786. [PubMed] [Google Scholar]
- 48.Mow VC. Structure and function relationships of the meniscus in the knee. In: Mow VC, Arnoczky S, Jackson D, editors. Knee Meniscus: Basic and Clinical Foundations. New York, NY: Raven Press; 1992. pp. 37–58. [Google Scholar]
- 49.McDevitt CA, Webber RJ. The ultrastructure and biochemistry of meniscal cartilage. Clin Orthop. 1990;252:8–18. [PubMed] [Google Scholar]
- 50.Arnoczky SP, Warren RF, Spivak JM. Meniscal repair using exogenous fibrin clot: an experimental study in dogs. J Bone Joint Surg Am. 1988;70:1209–1217. [PubMed] [Google Scholar]
- 51.Ingman A, Ghosh P, Taylor T. Variations of collagenous and non-collagenous proteins of human knee joint menisci with age and degeneration. Gerontology. 1974;20:212–233. doi: 10.1159/000212017. [DOI] [PubMed] [Google Scholar]
- 52.Peters TJ, Smillie IS. Studies on the chemical composition of the menisci of the knee joint with special reference to the horizontal cleavage lesion. Clin Orthop. 1972;86:245–252. doi: 10.1097/00003086-197207000-00037. [DOI] [PubMed] [Google Scholar]
- 53.Simon S. Anatomy, biology, and biomechanics of tendon, ligament, and meniscus. In: Simon S, Wilson J, editors. Orthopaedic Basic Science. Columbus, OH: American Academy of Orthopaedic Surgeons; 1994. [Google Scholar]
- 54.Bullough P, Munuera L, Murphy J, Weinstein AM. The strength of the menisci of the knee as it relates to their fine structure. J Bone Joint Surg Br. 1970;52:564–570. [PubMed] [Google Scholar]
- 55.Adams M, Hukins D. The extracellular matrix of the meniscus. In: Mow VC, Arnoczky S, Jackson D, editors. Knee Meniscus: Basic and Clinical Foundations. New York, NY: Raven Press; 1992. pp. 15–28. [Google Scholar]
- 56.Herwig J, Egner E, Buddecke E. Chemical changes of the human knee joint menisci in various stages of degeneration. Ann Rheum Dis. 1984;43:635–640. doi: 10.1136/ard.43.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Johnson LL, Johnson AL, Colquitt JA, Simmering MJ, Pittsley AW. Is it possible to make an accurate diagnosis based only on a medical history? A pilot study on women's knee joints. Arthroscopy. 1996;12:709–714. doi: 10.1016/s0749-8063(96)90175-9. [DOI] [PubMed] [Google Scholar]
- 58.Shelbourne KD, Martini DJ, McCarroll JR, Van Meter CD. Correlation of joint line tenderness and meniscal lesions in patients with acute anterior cruciate ligament tears. Am J Sports Med. 1995;23:166–169. doi: 10.1177/036354659502300207. [DOI] [PubMed] [Google Scholar]
- 59.Boeree NR, Ackroyd CE. Assessment of the menisci and cruciate ligaments: an audit of clinical practice. Injury. 1991;22:291–294. doi: 10.1016/0020-1383(91)90008-3. [DOI] [PubMed] [Google Scholar]
- 60.Anderson AF, Lipscomb AB. Clinical diagnosis of meniscal tears: description of a new manipulative test. Am J Sports Med. 1986;14:291–293. doi: 10.1177/036354658601400408. [DOI] [PubMed] [Google Scholar]
- 61.Dye SF, Vaupel GL, Dye CC. Conscious neurosensory mapping of the internal structures of the human knee without intraarticular anesthesia. Am J Sports Med. 1998;26:773–776. doi: 10.1177/03635465980260060601. [DOI] [PubMed] [Google Scholar]
- 62.Evans PJ, Bell GD, Frank C. Prospective evaluation of the McMurray test. Am J Sports Med. 1993;21:604–608. doi: 10.1177/036354659302100420. [DOI] [PubMed] [Google Scholar]
- 63.Fu FH, Baratz M. Meniscal injuries. In: De Lee JC, Drez DJ, editors. Orthopaedic Sports Medicine: Principles and Practice. Philadelphia, PA: WB Saunders; 1994. pp. 1146–1162. [Google Scholar]
- 64.Andrish JT. Meniscal injuries in children and adolescents: diagnosis and management. J Am Acad Orthop Surg. 1996;4:231–237. doi: 10.5435/00124635-199609000-00001. [DOI] [PubMed] [Google Scholar]
- 65.Annandale T. An operation for displaced semilunar cartilage. BMJ. 1885;1:779. doi: 10.1136/bmj.1.1268.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.DeHaven KE, Black KP, Griffiths HJ. Open meniscus repair: technique and two to nine year results. Am J Sports Med. 1989;17:788–795. doi: 10.1177/036354658901700612. [DOI] [PubMed] [Google Scholar]
- 67.DeHaven KE. Decision-making factors in the treatment of meniscus lesions. Clin Orthop. 1990;252:49–54. [PubMed] [Google Scholar]
- 68.Cooper DE, Arnoczky SP, Warren RF. Arthroscopic meniscal repair. Clin Sports Med. 1990;9:589–607. [PubMed] [Google Scholar]
- 69.Wickiewicz TL. Meniscal injuries in the cruciate-deficient knee. Clin Sports Med. 1990;9:681–694. [PubMed] [Google Scholar]
- 70.Warren RF. Meniscectomy and repair in the anterior cruciate ligament-deficient patient. Clin Orthop. 1990;252:55–63. [PubMed] [Google Scholar]
- 71.Gillquist J, Oretorp N. Arthroscopic partial meniscectomy: technique and long term results. Clin Orthop. 1982;167:29–33. [PubMed] [Google Scholar]
- 72.Northmore-Ball MD, Dandy DJ. Long term results of arthroscopic partial meniscectomy. Clin Orthop. 1982;167:34–42. [PubMed] [Google Scholar]
- 73.Cannon W. Arthroscopic meniscal repair. In: McGinty JB, Caspari RB, Jackson RW, editors. Operative Arthroscopy. New York, NY: Raven Press; 1993. pp. 237–251. [Google Scholar]
- 74.Cannon WD, Vittori J. Meniscal repair. In: Aichroth P, Cannon WD, editors. Knee Surgery: Current Practice. New York, NY: Raven Press; 1992. pp. 71–84. [Google Scholar]
- 75.Arnoczky SP, Warren RF. The microvasculature of the meniscus and its response to injury: an experimental study in the dog. Am J Sports Med. 1983;11:131–141. doi: 10.1177/036354658301100305. [DOI] [PubMed] [Google Scholar]
- 76.Weiss CB, Lundeberg M, Hamberg P, DeHaven KE, Gillquist J. Non-operative treatment of meniscal tears. J Bone Joint Surg Am. 1989;71:811–821. [PubMed] [Google Scholar]
- 77.DeHaven K, Stone R. Meniscal repair. In: Shahriaree H, editor. O'Connor's Textbook of Arthroscopic Surgery. Philadelphia, PA: Lippincott, Inc; 1983. pp. 327–338. [Google Scholar]
- 78.Henning CE. Current status of meniscal salvage. Clin Sports Med. 1990;9:567–576. [PubMed] [Google Scholar]
- 79.Morgan CD. The “all-inside” meniscus repair: technical note. Arthroscopy. 1991;7:120–125. doi: 10.1016/0749-8063(91)90093-d. [DOI] [PubMed] [Google Scholar]
- 80.Barrett GR, Richardson K, Koening V. T-Fix endoscopic meniscal repair technique and approach to different types of tears. Arthroscopy. 1995;11:245–251. doi: 10.1016/0749-8063(95)90077-2. [DOI] [PubMed] [Google Scholar]
- 81.Dervin GF, Downing KJ, Keene GC, McBride DG. Failure strengths of suture versus biodegradable arrow for meniscal repair: an in vitro study. Arthroscopy. 1997;13:296–300. doi: 10.1016/s0749-8063(97)90024-4. [DOI] [PubMed] [Google Scholar]
- 82.Henning C. Arthroscopic repair of meniscus tears. Orthopedics. 1983;6:1130–1132. doi: 10.3928/0147-7447-19830901-08. [DOI] [PubMed] [Google Scholar]
- 83.Rosenberg TD, Scott SM, Coward DB, et al. Arthroscopic meniscal repair evaluated with repeat arthroscopy. Arthroscopy. 1986;2:14–20. doi: 10.1016/s0749-8063(86)80005-6. [DOI] [PubMed] [Google Scholar]
- 84.Morgan CD, Casscells SW. Arthroscopic meniscus repair: a safe approach to the posterior horns. Arthroscopy. 1986;2:3–12. doi: 10.1016/s0749-8063(86)80003-2. [DOI] [PubMed] [Google Scholar]
- 85.Henning CE, Lynch MA, Yearout KM, Vequist SW, Stallbaumer RJ, Decker KA. Arthroscopic meniscal repair using exogenous fibrin clot. Clin Orthop. 1990;252:64–72. [PubMed] [Google Scholar]
- 86.Hashimoto J, Kurosake M, Yoshiya S, Hirohata K. Meniscal repair using fibrin sealant and endothelial cell growth factor: an experimental study in dogs. Am J Sports Med. 1992;20:537–541. doi: 10.1177/036354659202000509. [DOI] [PubMed] [Google Scholar]
- 87.Henning CE, Yearout KM, Vequist SW, Stallbaumer RJ, Decker KA. Use of the fascia sheath coverage and exogenous fibrin clot in the treatment of complex meniscal tears. Am J Sports Med. 1991;19:626–631. doi: 10.1177/036354659101900613. [DOI] [PubMed] [Google Scholar]
- 88.Fox JM, Rintz KG, Ferkel RD. Trephination of incomplete meniscal tears. Arthroscopy. 1993;9:451–455. doi: 10.1016/s0749-8063(05)80321-4. [DOI] [PubMed] [Google Scholar]
- 89.Zhang Z, Arnold JA, Williams T, McCann B. Repairs by trephination and suturing of longitudinal injuries in the avascular area of the meniscus in goats. Am J Sports Med. 1995;23:35–41. doi: 10.1177/036354659502300106. [DOI] [PubMed] [Google Scholar]
- 90.Henning CE, Clark JR, Lynch MA, Stallbaumer R, Yearout KM, Vequist SW. Arthroscopic meniscus repair with a posterior incision. Instr Course Lect. 1988;37:209–221. [PubMed] [Google Scholar]
- 91.Nakhostine M, Gershuni DH, Anderson R, Danzig LA, Weiner GM. Effects of abrasion therapy on tears in the avascular region of sheep menisci. Arthroscopy. 1990;6:280–287. doi: 10.1016/0749-8063(90)90057-k. [DOI] [PubMed] [Google Scholar]
- 92.Hanks GA, Gause TM, Sebastianelli WJ, O'Donnell CS, Kalenak A. Repair of peripheral meniscal tears: open versus arthroscopic technique. Arthroscopy. 1991;7:72–77. doi: 10.1016/0749-8063(91)90082-9. [DOI] [PubMed] [Google Scholar]
- 93.Mooney M, Rosenberg T. Meniscus repair: zone-specific technique. Sports Med Arthrosc Rev. 1993;1:136–144. [Google Scholar]
- 94.Ryu RK, Dunbar UH., 4th Arthroscopic meniscal repair with two year follow-up: a clinical review. Arthroscopy. 1988;4:168–173. doi: 10.1016/s0749-8063(88)80021-5. [DOI] [PubMed] [Google Scholar]
- 95.Oraevsky A, Esenaliev R, Letokhov V. Laser ablation. In: Miller JC, Haglund RF Jr, editors. Laser Ablation: Mechanisms and Applications. New York, NY: Springer-Verlag; 1991. [Google Scholar]
- 96.Schaffer JL, Dark M, Izkan I, et al. Mechanisms of meniscal tissue ablation by short pulse laser irradiation. Clin Orthop. 1995;310:30–36. [PubMed] [Google Scholar]
- 97.Forman SK, Oz MC, Lontz JF, Treat MR, Forman TA, Kiernan HA. Laser assisted fibrin clot soldering of human meniscus. Clin Orthop. 1995;310:37–41. [PubMed] [Google Scholar]
- 98.Buck BE, Malinin TI, Brown MD. Bone transplantation and human immunodeficiency virus: an estimate of risk of acquired immunodeficiency syndrome (AIDS) Clin Orthop. 1988;240:129–136. [PubMed] [Google Scholar]
- 99.Velteri DM, Warren RF, Wickiewicz TL, O'Brien SJ. Current status of allograft meniscal transplantations. Clin Orthop. 1994;303:44–55. [PubMed] [Google Scholar]
- 100.Garrett JC, Stevensen RN. Meniscal transplantation in the human knee: a preliminary report. Arthroscopy. 1991;7:57–62. doi: 10.1016/0749-8063(91)90079-d. [DOI] [PubMed] [Google Scholar]
- 101.Jackson DW, McDevitt CA, Simon TM, Arnoczky SP, Atwell EA, Silvino NJ. Meniscal transplantation using fresh and cryopreserved allografts: an experimental study in goats. Am J Sports Med. 1992;20:644–656. doi: 10.1177/036354659202000605. [DOI] [PubMed] [Google Scholar]
- 102.Keating E. Meniscal transplantation in goats: an experimental study. Trans Orthop Res Soc. 1988;12:147. [Google Scholar]
- 103.Urban WP, Jr, Nyland J, Caborn DNM, Johnson DL. The radiographic position of medial and lateral meniscal horns as a basis for meniscal reconstruction. Arthroscopy. 1999;15:147–154. doi: 10.1053/ar.1999.v15.0150141. [DOI] [PubMed] [Google Scholar]
- 104.Maitra RS, Miller MD, Johnson DL. Meniscal reconstruction, part I: indications, techniques, and graft considerations. Am J Orthop. 1999;28:213–218. [PubMed] [Google Scholar]
- 105.Kuhn JE, Wojtys EM. Allograft meniscus transplantation. Clin Sports Med. 1996;15:537–556. [PubMed] [Google Scholar]
- 106.Wilcox TR, Goble EM. Indications for meniscal allograft reconstruction. Am J Knee Surg. 1996;9:35–36. [PubMed] [Google Scholar]
- 107.Arnoczky S, Warren RF, McDevitt C. Meniscal replacement using a cryopreserved allograft: an experimental study in the dog. Clin Orthop. 1990;252:121–128. [PubMed] [Google Scholar]
- 108.Arnoczky S, Milachowsky K. Meniscal allografts: where do we stand? In: Ewing JW, editor. Articular Cartilage and Knee Joint Function: Basic Science and Arthroscopy. New York, NY: Raven Press; 1990. pp. 129–136. [Google Scholar]
- 109.Rodkey WG, Steadman JR, Li ST. A clinical study of collagen meniscus implants to restore the injured meniscus. Clin Orthop. 1999;367:S281–S292. doi: 10.1097/00003086-199910001-00027. [DOI] [PubMed] [Google Scholar]
- 110.Jokl P, Stull PA, Lynch JK, Vaughan V. Independent home versus supervised rehabilitation following arthroscopic knee surgery: a prospective randomized trial. Arthroscopy. 1989;5:298–305. doi: 10.1016/0749-8063(89)90145-x. [DOI] [PubMed] [Google Scholar]
- 111.Moffet H, Richards CL, Malouin F, Bravo G, Paradis G. Early and intensive physiotherapy accelerates recovery postarthroscopic meniscectomy: results of a randomized controlled study. Arch Phys Med Rehabil. 1994;75:415–426. doi: 10.1016/0003-9993(94)90165-1. [DOI] [PubMed] [Google Scholar]
- 112.Matthews P, St-Pierre DM. Recovery of muscle strength following arthroscopic meniscectomy. J Orthop Sports Phys Ther. 1996;23:18–26. doi: 10.2519/jospt.1996.23.1.18. [DOI] [PubMed] [Google Scholar]
- 113.St-Pierre DM. Rehabilitation following arthroscopic meniscectomy. Sports Med. 1995;20:338–347. doi: 10.2165/00007256-199520050-00005. [DOI] [PubMed] [Google Scholar]
- 114.Zhang ZN, Xu YK, Zhang WM, Zhou ZH, Ou SH. Suture and immobilization of acute peripheral injuries of the meniscus in rabbits. Arthroscopy. 1986;2:227–233. doi: 10.1016/s0749-8063(86)80077-9. [DOI] [PubMed] [Google Scholar]
- 115.Newman AP, Anderson DR, Daniels AU, Dales MC. Mechanics of the healed meniscus in a canine model. Am J Sports Med. 1989;17:164–175. doi: 10.1177/036354658901700205. [DOI] [PubMed] [Google Scholar]
- 116.Eriksson E, Haggmart T. Comparison of isometric muscle training and electrical stimulation supplementing isometric muscle training in recovery after major knee ligament surgery: a preliminary report. Am J Sports Med. 1979;7:169–171. doi: 10.1177/036354657900700305. [DOI] [PubMed] [Google Scholar]
- 117.Jakob RP, Staubli HU, Zuber K, Esser M. The arthroscopic meniscal repair. Am J Sports Med. 1988;16:137–142. doi: 10.1177/036354658801600208. [DOI] [PubMed] [Google Scholar]
- 118.Fowler P, Pompan D. Rehabilitation after meniscal repair. Techniq Orthop. 1993;8:37–39. [Google Scholar]
- 119.Salter RB, Simmonds DF, Malcolm BW, Rumble EJ, MacMichael D, Clements ND. The biological effect of continuous passive motion on the healing of full-thickness defects in articular cartilage: an experimental investigation in the rabbit. J Bone Joint Surg Am. 1980;62:1232–1251. [PubMed] [Google Scholar]
- 120.Shelbourne KD, Patel DV, Adsit WS, Porter DA. Rehabilitation after meniscal repair. Clin Sports Med. 1996;15:595–612. [PubMed] [Google Scholar]
- 121.Irrgang JJ, Pezzullo D. Rehabilitation following surgical procedures to address articular cartilage lesions in the knee. J Orthop Sports Phys Ther. 1998;28:232–240. doi: 10.2519/jospt.1998.28.4.232. [DOI] [PubMed] [Google Scholar]
- 122.DeHaven K, Bronstein R. Open meniscus repair. Op Techniq Sports Med. 1994;2:172–175. [Google Scholar]
- 123.Sommerlath K. The prognosis of repaired and intact menisci in unstable knees: a comparative study. Arthroscopy. 1988;4:93–95. doi: 10.1016/s0749-8063(88)80072-0. [DOI] [PubMed] [Google Scholar]
- 124.Barber FA. Accelerated rehabilitation for meniscus repairs. Arthroscopy. 1994;10:206–210. doi: 10.1016/s0749-8063(05)80095-7. [DOI] [PubMed] [Google Scholar]
- 125.Barber FA, Click SD. Meniscus repair rehabilitation with concurrent anterior cruciate reconstruction. Arthroscopy. 1997;13:433–437. doi: 10.1016/s0749-8063(97)90120-1. [DOI] [PubMed] [Google Scholar]
- 126.Mintzer CM, Richmond JC, Taylor J. Meniscal repair in the young athlete. Am J Sports Med. 1998;26:630–633. doi: 10.1177/03635465980260050601. [DOI] [PubMed] [Google Scholar]
- 127.Tovin BJ, Wolf SL, Greenfield BH, Crouse J, Woodfin BA. Comparison of the effects of exercise in water and on land on the rehabilitation of patients with intra-articular anterior cruciate ligament reconstructions. Phys Ther. 1994;74:710–719. doi: 10.1093/ptj/74.8.710. [DOI] [PubMed] [Google Scholar]
- 128.Kuhne C, Zirkel A. Accelerated rehabilitation following patellar tendon autograft anterior cruciate ligament reconstruction using the aqua-jogging protocol: a primary study. Sports Exerc Inj. 1996;2:15–23. [Google Scholar]