Abstract
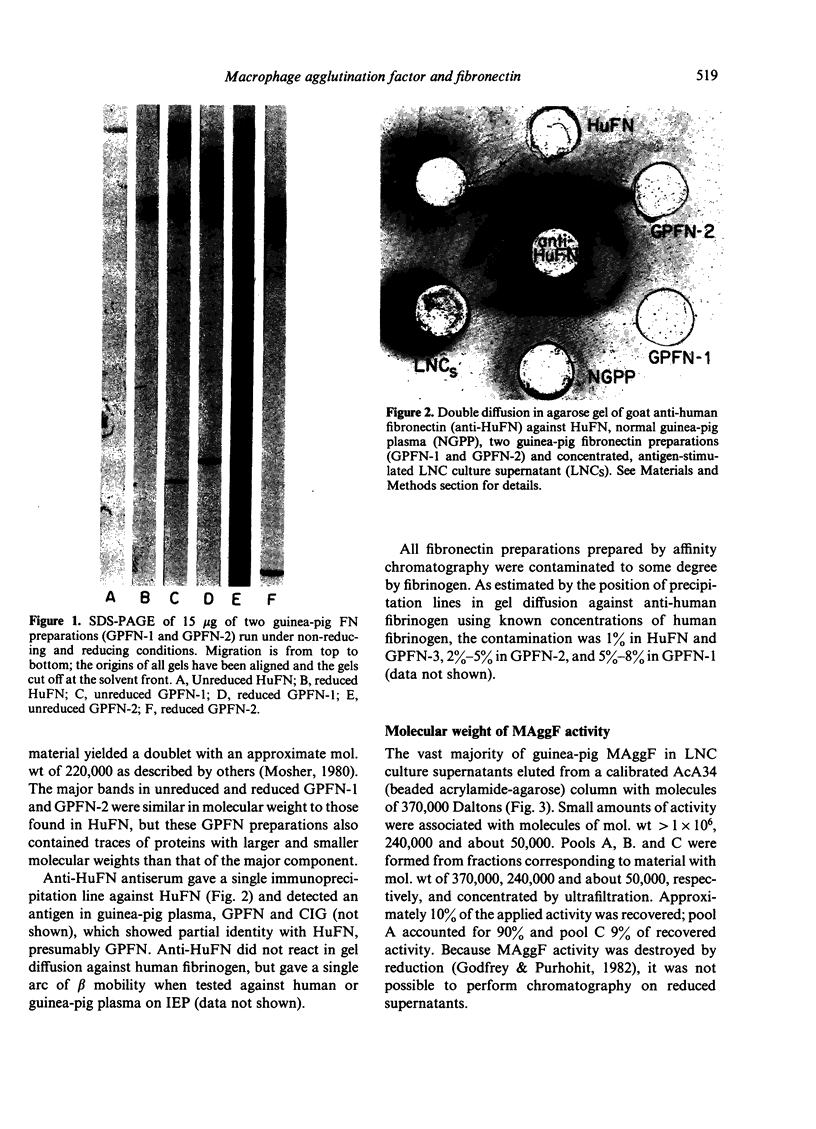
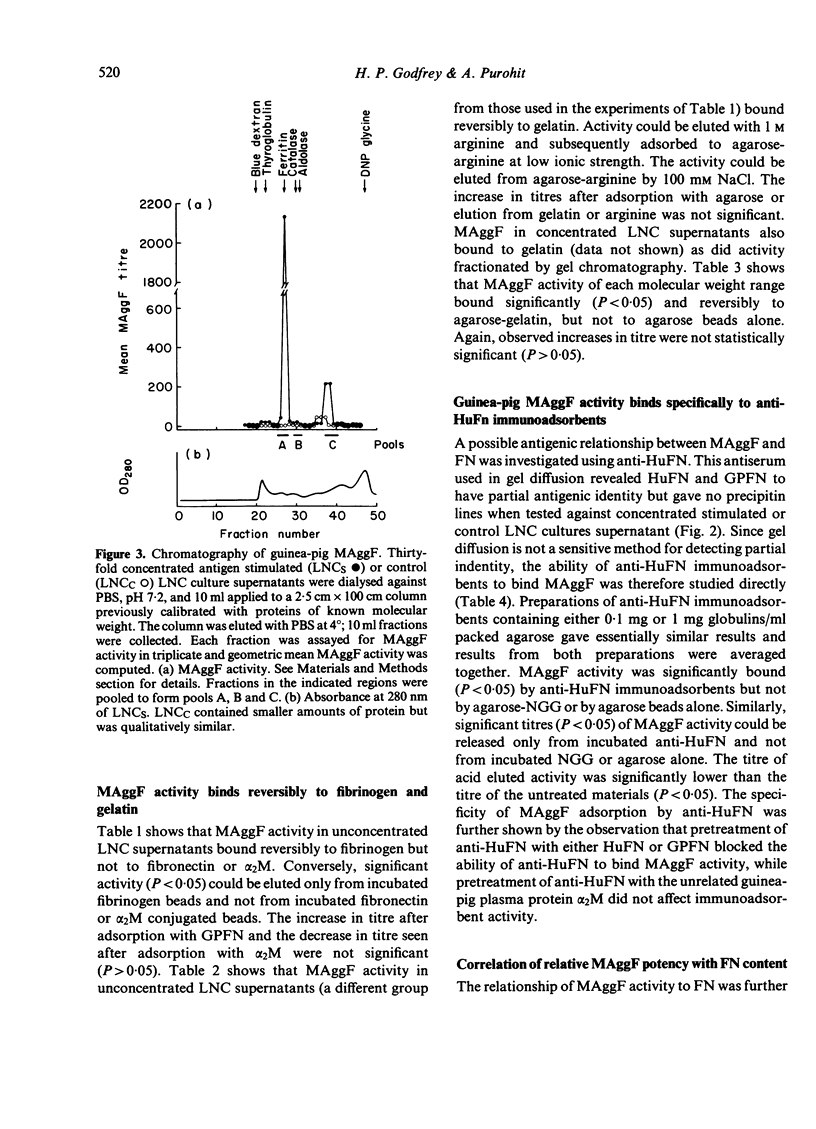
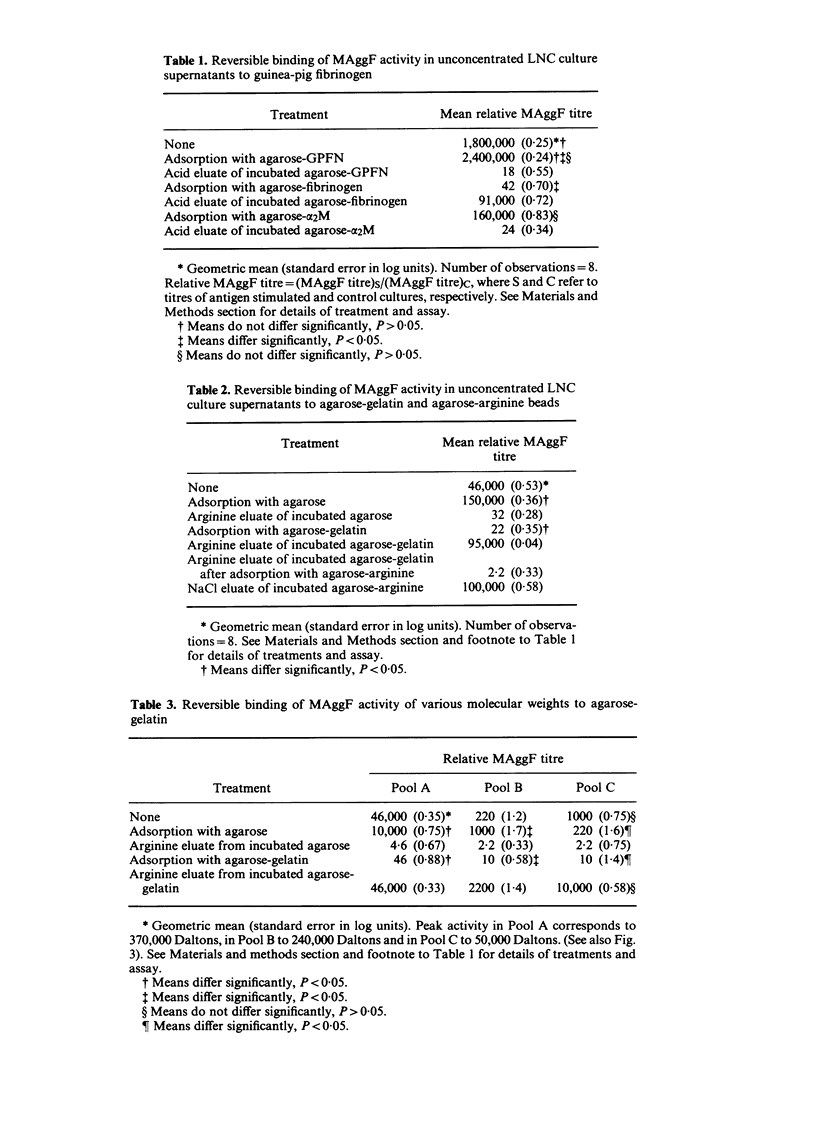
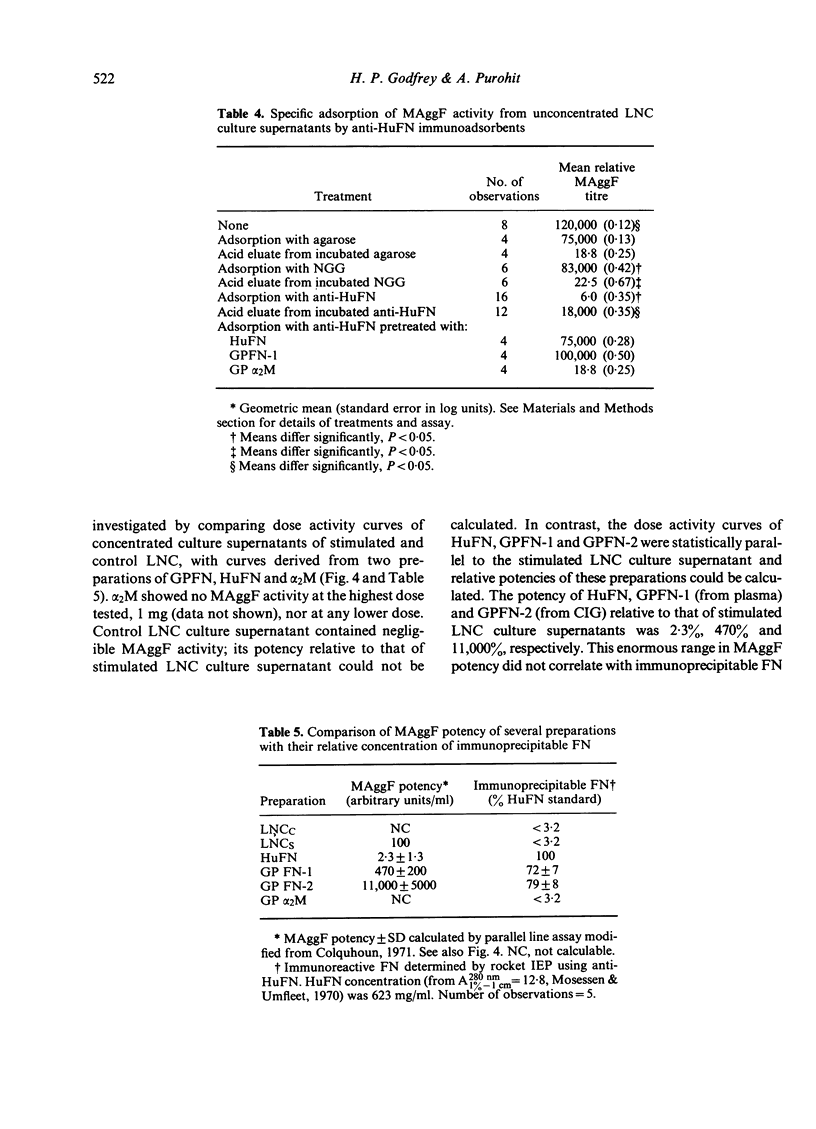
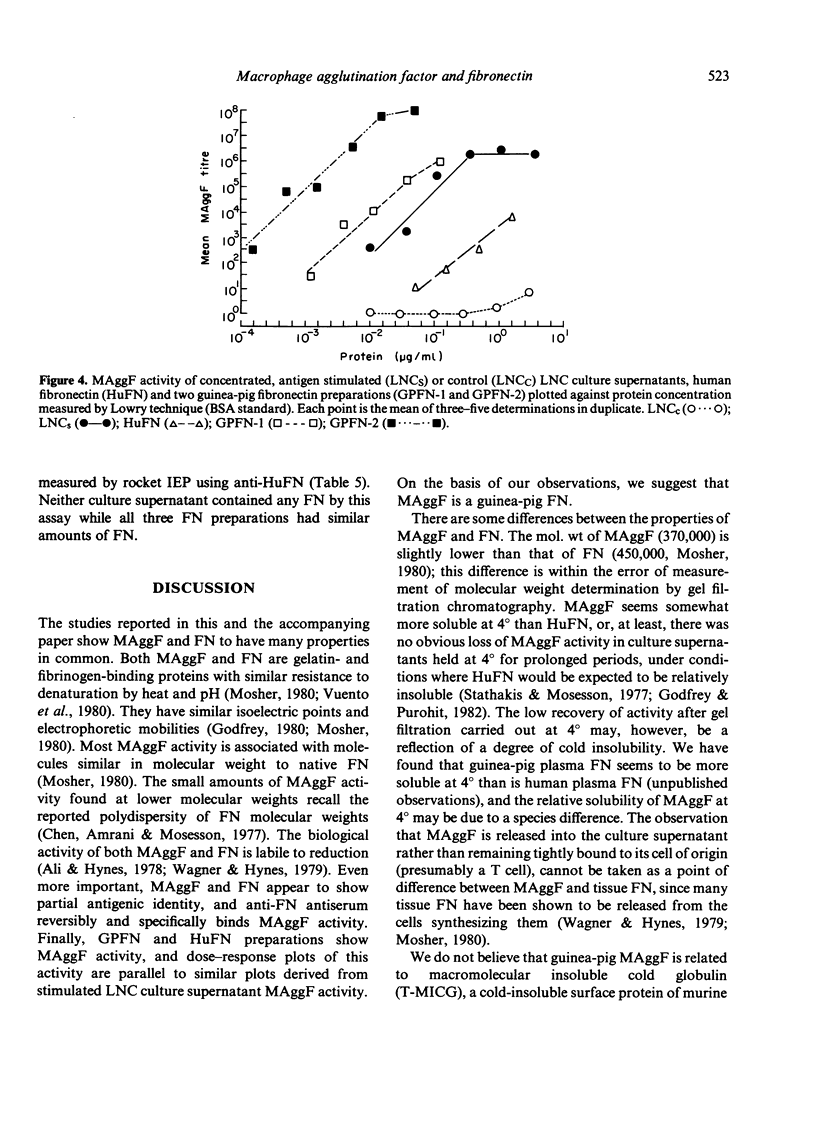
Macrophage agglutination factor (MAggF) is a T-cell-dependent guinea-pig lymphokine with pH stability, heat stability and isoelectric point similar to fibronectin (see preceeding paper for details). Further observations confirm the similarity between MAggF and fibronectin. MAggF in unconcentrated lymph node cell culture supernatants bound reversibly to gelatin and fibrinogen. On gel filtration chromatography, most MAggF activity in a pooled concentrated lymphokine preparation was associated with molecules of 370,000 Daltons; lesser amounts of activity were found at 240,000 and 50,000 Daltons. All molecular weight forms of MAggF bound reversibly to gelatin. Guinea-pig plasma fibronectin prepared by affinity chromatography over gelatin had a molecular weight of about 450,000, a sub-unit on reduction of about 240,000 Daltons, and showed partial antigenic identity with human plasma fibronectin. Human and guinea-pig fibronectin preparations showed MAggF activity when tested using guinea-pig peritoneal macrophages, but their potencies relative to a culture supernatant standard did not correlate with the content of immunoprecipitable fibronectin measured by anti-human fibronectin antiserum. However, anti-human fibronectin immunoadsorbents specifically and reversibly bound MAggF activity in culture supernatants. On the basis of our observations, we suggest MAggF is a guinea-pig tissue fibronectin.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen A. B., Amrani D. L., Mosesson M. W. Heterogeneity of the cold-insoluble globulin of human plasma (CIg), a circulating cell surface protein. Biochim Biophys Acta. 1977 Aug 23;493(2):310–322. doi: 10.1016/0005-2795(77)90187-8. [DOI] [PubMed] [Google Scholar]
- Clark R. A., Dvorak H. F., Colvin R. B. Fibronectin in delayed-type hypersensitivity skin reactions: associations with vessel permeability and endothelial cell activation. J Immunol. 1981 Feb;126(2):787–793. [PubMed] [Google Scholar]
- Godfrey H. P., Geczy C. L. Guinea pig macrophage agglutination factor is antigenically distinct from migration inhibition factor and immunoglobulin. J Immunol. 1978 Oct;121(4):1428–1431. [PubMed] [Google Scholar]
- Godfrey H. P., Gell P. G. Cellular and molecular events in the delayed-onset hypersensitivities. Rev Physiol Biochem Pharmacol. 1978;84:1–92. doi: 10.1007/BFb0030490. [DOI] [PubMed] [Google Scholar]
- Godfrey H. P. Hapten-specific responses to contact sensitizers. Use of fluorodinitrobenzene to elicit migration inhibition and macrophage agglutination factors from lymph node cells of contact-sensitive guinea-pigs. Immunology. 1976 May;30(5):685–694. [PMC free article] [PubMed] [Google Scholar]
- Godfrey H. P., Koch C. Ability of an anti-T-cell serum to dissociate two features of cellular hypersensitivity in the guinea-pig. Immunology. 1980 Jun;40(2):247–253. [PMC free article] [PubMed] [Google Scholar]
- Godfrey H. P., Purohit A. Characterization of a guinea-pig lymphokine, macrophage agglutination factor. Immunology. 1982 Jul;46(3):507–513. [PMC free article] [PubMed] [Google Scholar]
- Hauptman S. P., Kansu E., Serno M., Godfrey S. Human macromolecular insoluble cold globulin (MICG). I. T-cell origin of T-MICG and null cell origin of N-MICG. J Exp Med. 1979 Jan 1;149(1):158–171. doi: 10.1084/jem.149.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauptman S. P. Macromolecular insoluble cold globulin (MICG): a novel protein from mouse lymphocytes--I. Isolation and characterization. Immunochemistry. 1978 Jul;15(7):415–422. doi: 10.1016/0161-5890(78)90068-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- March S. C., Parikh I., Cuatrecasas P. A simplified method for cyanogen bromide activation of agarose for affinity chromatography. Anal Biochem. 1974 Jul;60(1):149–152. doi: 10.1016/0003-2697(74)90139-0. [DOI] [PubMed] [Google Scholar]
- Mosesson M. W., Umfleet R. A. The cold-insoluble globulin of human plasma. I. Purification, primary characterization, and relationship to fibrinogen and other cold-insoluble fraction components. J Biol Chem. 1970 Nov 10;245(21):5728–5736. [PubMed] [Google Scholar]
- Mosher D. F. Fibronectin. Prog Hemost Thromb. 1980;5:111–151. [PubMed] [Google Scholar]
- Postlethwaite A. E., Kang A. H. Guinea pig lymphocyte-derived macrophage aggregation factor: its separation from macrophage migration inhibitory factor. J Immunol. 1976 Nov;117(5 Pt 1):1651–1655. [PubMed] [Google Scholar]
- Rouveix B., Badenoch-Jones P., Turk J. L. A sensitive technique for measuring specific macrophage aggregation: a comparison with macrophage migration inhibition, for the detection of lymphokine activity. Immunology. 1979 Mar;36(3):589–594. [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E., Engvall E. Immunochemical and collagen-binding properties of fibronectin. Ann N Y Acad Sci. 1978 Jun 20;312:178–191. doi: 10.1111/j.1749-6632.1978.tb16802.x. [DOI] [PubMed] [Google Scholar]
- Stathakis N. E., Mosesson M. W. Interactions among heparin, cold-insoluble globulin, and fibrinogen in formation of the heparin-precipitable fraction of plasma. J Clin Invest. 1977 Oct;60(4):855–865. doi: 10.1172/JCI108840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto Y., Helsel W. E., Wahl S. M. Macrophage production of fibronectin, a chemoattractant for fibroblasts. J Immunol. 1981 Aug;127(2):673–678. [PubMed] [Google Scholar]
- Vuento M., Salonen E., Salminen K., Pasanen M., Stenman U. K. Immunochemical characterization of human plasma fibronectin. Biochem J. 1980 Dec 1;191(3):719–727. doi: 10.1042/bj1910719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuento M., Vaheri A. Purification of fibronectin from human plasma by affinity chromatography under non-denaturing conditions. Biochem J. 1979 Nov 1;183(2):331–337. doi: 10.1042/bj1830331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D. D., Hynes R. O. Domain structure of fibronectin and its relation to function. Disulfides and sulfhydryl groups. J Biol Chem. 1979 Jul 25;254(14):6746–6754. [PubMed] [Google Scholar]