Abstract
Objectives:
To provide the reader with an overview of the many causes of sudden cardiac death in young athletes and to present various strategies for preparticipation cardiovascular screening.
Data Source:
A MEDLINE search using the phrase sudden cardiac death and the key word athlete for the years 1980 to 2000.
Data Synthesis:
Sudden cardiac death is a rare event in athletics. More than 20 different causes have been described, but most cases result from a few distinct entities. Most afflicted athletes have no symptoms before death. Many attempts have been made to detect those at risk for sudden cardiac death before athletic participation. At this time, a thorough history and physical examination are the most efficient screening methods for detecting cardiovascular abnormalities. Studies show that the current status of preparticipation cardiovascular screening of high school and college athletes nationwide is poor.
Conclusions and Recommendations:
The use of diagnostic tests to screen for cardiovascular abnormalities is ineffective and inefficient. The most prudent and effective methods of preparticipation screening for cardiovascular abnormalities at this time are a history and physical examination in accordance with the American Heart Association guidelines. Athletic trainers must ensure that their institutions comply with these minimum standards.
Keywords: sudden cardiac death, hypertrophic cardiomyopathy, preparticipation athletic evaluation, coronary artery anomalies
The sudden, unexpected death of a young athlete is a tragedy unparalleled in sports. Aside from the grief of friends and family members, shock waves reverberate as the community, institution (high school, college, or professional organization), and sports medicine team all cope with the death. Instinctively, those involved wonder what intervention might have prevented the death. Occasionally, the search for answers may spawn a parallel search to assign blame.1 Every case of sudden death in a young athlete garners large amounts of media attention. Indeed, such publicity may influence the public perception as to the frequency of these events. Although sudden cardiac death (SCD) is a long-recognized entity, the deaths of Hank Gathers and Reggie Lewis in the early 1990s focused new attention on the condition.1–6
Fatal sport-related injuries can result from head and cervical spine trauma, but most sudden deaths in athletes are cardiac in origin.7,8 An enormous amount of research has been generated during the past 10 years evaluating the causes and events surrounding SCD and potential screening mechanisms for identifying those at risk. Concomitantly, the 1990s ushered in a renewed interest and changing focus in the preparticipation athletic evaluation (PAE), as researchers and sports medicine practitioners implemented a variety of methods to improve the long-standing and often controversial “sports physical.” Currently, these approaches vary, but several thorough and practical evaluation models have been proposed.9–15
The purpose of this article is to present the athletic trainer with a practical base of knowledge from which to develop the cardiovascular portion of the PAE for young competitive athletes. With this knowledge, the athletic trainer, in conjunction with the team physician, can review their institution's current PAE format. If necessary, changes can be implemented to develop a more prudent and effective means of screening for potential cardiac anomalies. Although SCD affects all age groups and can occur in any setting, my discussion will focus on athletes younger than 35 years.
INCREASING AWARENESS
The first recorded sudden death of an athlete was that of Pheidippides, a young long-distance messenger, in 490 bc. On arrival in Athens, he reported the defeat of the Persian army and then fell dead.15 The more recent deaths of several well-known athletes have brought SCD into the public consciousness. Athletes who experienced SCD include marathon runner Jim Fixx (1984), Olympic volleyball player Flo Hyman (1986), former basketball star Pete Maravich (1988), college basketball star Hank Gathers (1990), professional basketball All-Star Reggie Lewis (1993), and Olympic figure skating champion Sergei Grinkov (1995). All died from cardiac causes.
The deaths of Hank Gathers and Reggie Lewis spurred an increased awareness of SCD in the sports medicine community and the public.1,2,16 As a student athletic trainer in the early 1990s, I recall the impact of the Gathers' tragedy as SCD became a frequent subject of athletic training room and classroom discussions. What was once thought of as an exceedingly uncommon occurrence at the time took on the appearance of a commonplace event. Fortunately, SCD remains rare.
EPIDEMIOLOGY
Although not a universally accepted definition, SCD can be considered a nontraumatic, nonviolent, unexpected death due to cardiac causes within 1 hour of the onset of symptoms.17 The exact incidence of SCD is difficult to ascertain, because many studies have relied on the self-reporting of physicians and media accounts of deaths.7,8 The National Federation of State High School Associations estimates 10 to 25 cases of SCD per year in individuals younger than 30 years.18 Although not representative of the general population, given the presumed state of good health, data compiled from 1965 to 1985 show the incidence of sudden death to be only 1 per 735 000 screened US Air Force recruits between 17 and 28 years of age.19 A study of Minnesota high schools revealed 3 individuals succumbing to SCD during a 12-year period, translating to a risk of 1 death per 200 000 athletes per year.20 The incidence of sudden death during exercise in unscreened men younger than 30 years was estimated to be 1 death per 280 000 men per year in Rhode Island.21
In a landmark study, Maron et al7 detailed the clinical, demographic, and pathologic profiles of 134 young, competitive athletes experiencing SCD from 1985 through 1995. The mean age was 17 years (range, 12 to 40 years); 90% were male and 44% were black. Basketball and football players accounted for 68% of the deaths. The National Center for Catastrophic Sports Injury Research8 identified 160 athletes dying from nontraumatic causes (78% of deaths were from cardiac causes) in high school and college sports between June 1983 and June 1993. The estimated death rate of male athletes was 5-fold higher than for female athletes (7.47 versus 1.33 per 1 000 000 athletes per year), and 65% of the deaths occurred during participation in basketball or football. Interestingly, male college athletes had twice the estimated death rate of their high school counterparts (14.5 versus 6.6 per 1 000 000 athletes per year).
THE ATHLETE'S HEART AND CARDIOVASCULAR RESPONSE TO EXERCISE
A brief overview of adaptive cardiovascular physiology is necessary before a discussion of the causes of SCD. Exercise results in both hemodynamic and electrophysiologic changes within normal myocardial tissue. During intense aerobic exercise, the oxygen consumption of muscle tissue increases markedly, and cardiac output must rise to meet the demands. Over time, aerobic training results in increased left ventricular mass, increased heart rate during exercise (decreased resting heart rate), increased ventricular stroke volume, and increased cardiac output, among other effects.22
First demonstrated in 1935, physiologic hypertrophy of the heart in response to cardiovascular conditioning is prevalent among well-trained athletes.23 The amount of adaptation depends on the intensity of training and can be assessed through changes on physical examination, electrocardiography (ECG), and echocardiography.15,23,24 The left ventricle hypertrophy is typically symmetric (ie, hypertrophy of the septum and left ventricular free wall is equal), plateaus early in conditioning, and rapidly diminishes back to baseline within weeks of inactivity.22
The electrophysiologic changes brought on by exercise are also enhanced by emotion and competitive stress. The release of circulating catecholamines stimulates heart rate, myocardial contractility, and blood pressure, all resulting in increased myocardial oxygen consumption. Such changes may perturb underlying myocardial ischemia and trigger a variety of cardiac arrhythmias if a pre-existing abnormality is present. In one study,7 90% of athletes collapsed during or immediately after exercise, highlighting this vulnerable period.
CAUSES OF SUDDEN CARDIAC DEATH
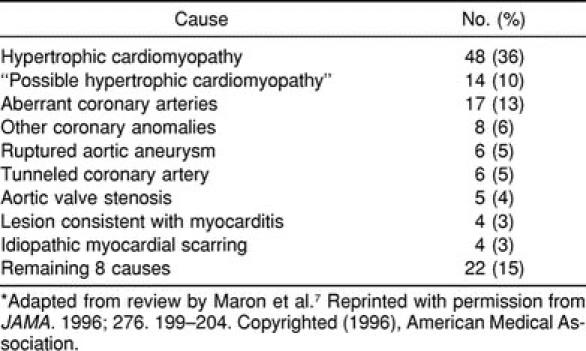
More than 20 pathologic entities have been identified as causes of SCD in young athletes (Table 1). However, a few lesions are responsible for most deaths. McCaffrey et al25 reviewed 7 studies and found hypertrophic cardiomyopathy (HCM) responsible for 24% of the deaths, whereas coronary artery abnormalities were present in 18% of patients. Coronary artery disease and myocarditis accounted for 14% and 12% of cases, respectively. The review by Maron et al7 found HCM or “possible HCM” to account for 46% of all deaths, with coronary artery anomalies leading to an additional 19% of SCD cases (Table 2). Other studies have found HCM responsible for nearly half of all deaths.26,27
Table 1.
Conditions Linked to Cases of Sudden Cardiac Death*
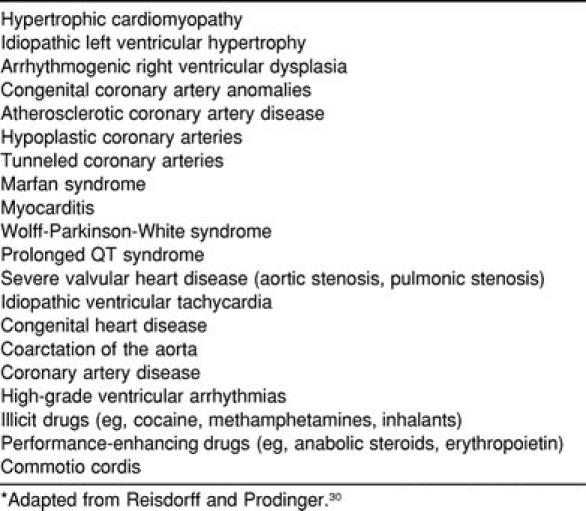
Table 2.
Most Common Causes of Sudden Cardiac Death in 134 Athletes*
Although a thorough discussion of all causes of SCD is beyond the scope of this review, I will present an overview of the most common causes and those less common causes that lend themselves to clinical detection through preparticipation screening.
Hypertrophic Cardiomyopathy
Although rare in the general population (0.1% to 0.2% prevalence),28 HCM is the most common cause of SCD in young athletes.7,25–27 It was HCM that claimed the life of Hank Gathers in what has certainly been the most notorious case of SCD.1 Typically, HCM is inherited as an autosomal dominant condition; more than 100 individual genetic defects can result in the characteristic pathologic findings.16 Sixty percent of individuals with HCM have an affected first-degree relative.26
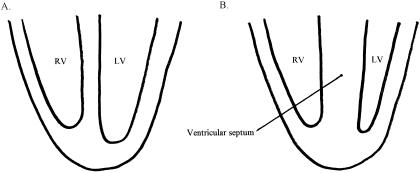
On autopsy, patients with HCM are found to have a larger-than-normal heart with a distinctively enlarged left ventricle. The total mass of the left ventricle is increased without compensatory dilatation of the chamber; thus, ventricular filling is decreased during diastole. The thickness of the ventricular septal wall may be markedly increased, from 15 to 50 mm, with less than 13 mm being considered normal, even in a trained athlete.22 Hypertrophy of the ventricular septum is disproportionate to that of the left ventricular free wall, an asymmetry not seen in physiologic hypertrophy (Figure 1). In addition to the increased size, the asymmetric thickening of the septum may act as an obstruction to the flow of blood into the aorta during diastole. Microscopic changes include abnormalities of the small arteries and “myocardial disarray,” a bizarre arrangement of muscle cells with diffuse interstitial fibrosis.29
Figure 1.
Cross-sectional views. A, Normal ventricular septal anatomy. B, Asymmetric septal hypertrophy found in hypertrophic cardiomyopathy. RV indicates right ventricle; LV, left ventricle.
The hallmark physical examination finding in HCM is a systolic murmur that decreases in intensity with the athlete in the supine position. This contrasts with functional outflow murmurs common in athletes, which increase in intensity with lying down. Approximately 90% of patients with HCM have abnormal ECG results.30 In many, but not all, cases, HCM can be diagnosed by echocardiographic findings of marked, asymmetric left ventricle wall thickening, diminished left ventricle chamber size, and abnormal diastolic filling.22
Despite the aforementioned information, individuals typically present with SCD as their first and only symptom of HCM. A few may have a family history of a sudden or unexplained death. In one study,7 just 10 (21%) of 48 athletes who died of HCM had signs or symptoms of cardiac disease (chest pain, exertional dyspnea, syncope, dizziness) before death. The mechanism of death is not fully understood but is most likely secondary to a malignant arrhythmia arising within the abnormal myocardial fibers. Syncope may result from either dynamic obstruction of the left ventricular outflow tract or a nonsustained arrhythmia.
Idiopathic Left Ventricular Hypertrophy
Idiopathic left ventricular hypertrophy (ILVH) is an ill-defined and poorly understood condition responsible for up to 10% of SCD cases. Although ILVH is difficult to diagnose during life, on autopsy the heart shows symmetric enlargement, unlike that seen in HCM but with hypertrophy far in excess of that seen in trained athletes.27 In addition, the coronary arteries and the myocardial tissue are normal.30 The family history reveals no HCM or prior events of SCD. At this time, the cause of ILVH is undetermined. Possible causes include adaptation to undiagnosed systemic hypertension, extreme physiologic hypertrophy,16 and a nonfamilial variant of HCM.30
Coronary Artery Anomalies
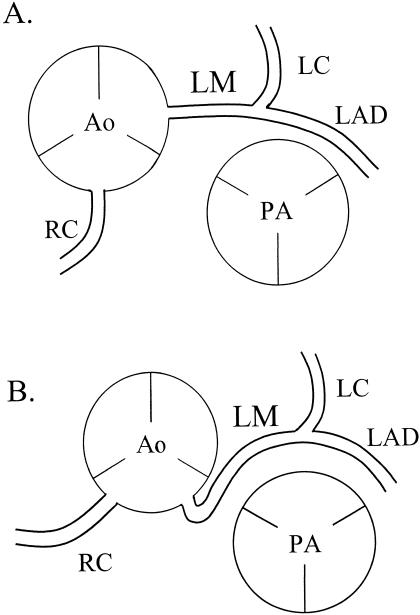
A variety of congenital coronary artery anomalies combine to represent the second leading cause of SCD in young athletes. The most common abnormality consists of the left main coronary artery arising from the right sinus of Valsalva (Figure 2). The artery comes off the sinus at an acute angle, which is thought to contribute to diminished blood flow as the aorta dilates during exercise.31 The aberrant artery then courses between the aorta and pulmonary trunk, making it prone to compression as the great vessels enlarge with the increased cardiac output of exercise.32 Other anomalies have been described, including reports of single coronary arteries.22
Figure 2.
A, Normal coronary artery anatomy. B, Most common congenital cardiac artery anomaly with left main (LM) coronary artery arising from the right sinus of Valsalva and coursing between the aorta (Ao) and pulmonary artery (PA). This anatomic variation also results in the right coronary artery's (RC) originating in a different region than normal, but its function is not affected. LC indicates left coronary artery; LAD, left anterior descending artery.
Only about one third of affected individuals are thought to be symptomatic (experiencing angina, syncope, or exertional dyspnea) before SCD.33 However, 10 of 12 athletes with coronary artery anomalies in the United States and Italy had symptoms before death.34 All 9 athletes who underwent ECG testing had normal results, including 6 individuals with normal exercise stress test results. The mechanism of SCD in all cases is thought to be an arrhythmia triggered by myocardial ischemia or infarction. Some cases may be suspected on echocardiography, but a definitive diagnosis is made by coronary angiography, computed tomography, or magnetic resonance imaging.
Myocarditis
Acute myocarditis is an inflammatory condition of infectious origin. Coxsackie B virus causes more than 50% of all cases, but a variety of pathogens have been implicated.35 Affected individuals may present with dyspnea, orthopnea, cough, and exercise intolerance. When present, such symptoms are often overshadowed or preceded by symptoms of viral illness such as vomiting, fever, nausea, diarrhea, and myalgias. However, many individuals are asymptomatic, and SCD may be the only presenting sign.15,22,30 The infected myocardium becomes inflamed, creating an unstable site where a potentially terminal arrhythmia may arise. In other cases, involvement of the conduction system may lead to a fatal heart block.
Myocarditis is suspected based on the clinical symptoms in conjunction with physical examination findings of congestive heart failure (eg, audible S3 gallop, distended neck veins, peripheral edema, hepatomegaly). Although chest radiographs, ECG, and echocardiography may suggest the disease, diagnosis can only be confirmed by myocardial biopsy. Maron et al7 described 4 cases of SCD secondary to acute myocarditis and 4 other deceased athletes with pathologic findings of isolated idiopathic myocardial scarring. These cases may represent an arrhythmogenic potential for healed myocarditis.36
Marfan Syndrome
Marfan syndrome is an autosomal, dominantly inherited connective tissue disorder occurring in about 1 in 10 000 people. Affected individuals are at increased risk for SCD as the result of progressive dilatation of the aortic root, culminating in complete dissection or rupture of the aorta with subsequent mediastinal hemorrhage, pericardial tamponade, coronary artery dissection, or acute aortic insufficiency with rapid congestive heart failure.30 Aortic root dilatation results from cystic medial necrosis, a condition that weakens the walls of the aorta because of a decreased number of elastic fibers.
The diagnosis of Marfan syndrome is based on clinical criteria (Table 3), although genetic testing may be appropriate in families with several affected members. Clinical features include tall stature, long and thin limbs (dolichostenomelia), an arm span substantially greater than height, diminished upper body–to–lower body ratio, and long, thin facies. Additional findings include anterior thorax abnormalities (pectus excavatum or carinatum), hyperextensible joints, and the ability to significantly overlap the thumb and fifth digit while encircling the thin wrist.37 The defective connective tissue also may place those affected at risk for a dislocation of the eye lens.
Table 3.
History and Physical Examination Findings Suggestive of Marfan Syndrome*

Electrophysiologic Abnormalities
Abnormalities of the conduction system may lead to fatal cardiac arrhythmias. Their incidence is likely underreported since autopsy findings may be inconclusive.7 The 2 most commonly encountered abnormalities in the general population are Wolff-Parkinson-White syndrome (WPW) and long QT syndrome (LQTS). WPW represents an accessory conduction pathway within the myocardium, which, when triggered, results in symptomatic, but typically benign, atrial or ventricular arrhythmias. Rarely, these arrhythmias may degenerate into ventricular fibrillation and prove fatal. Often, WPW can be recognized by specific ECG findings, but a significant number of accessory pathways are “concealed,” that is, the ECG results are normal despite the presence of the pathway. The condition may be treated with medication, but the treatment of choice in competitive athletes is radiofrequency ablation of the accessory pathway.
An inherited cardiac disorder, LQTS occurs in about 1 in 10 000 individuals, with 60% of those having a positive history of LQTS or SCD in a family member.38 Approximately 60% of patients present with symptoms related to physical activity or strong emotional response, primarily syncope, seizures, or heart palpitations. One third of previously “healthy” young adults present with SCD.38 The mechanism of demise is a distinctive fatal arrhythmia triggered by catecholamine release. The ECG results are abnormal in nearly all affected individuals. Treatment generally involves β-blocker medication (sometimes in conjunction with permanent cardiac pacing) and avoidance of intense physical exertion.
Other Causes
A number of more rare causes have also been implicated in SCD. Aortic stenosis and mitral valve prolapse are often described as risk factors for SCD, but the mechanism of death in each is uncertain. Mitral valve prolapse is a relatively common condition, and its association with SCD is controversial. Athletes who had congenital heart malformations, such as tetralogy of Fallot, repaired in infancy are at risk for fatal arrhythmias. A number of illicit drugs have also been implicated in SCD. Cocaine abuse may cause local ischemia and infarction due to vasospasm, whereas inhalant use has resulted in fatal arrhythmias. Additional deaths have been linked to performance-enhancing agents such as erythropoietin and anabolic steroids.15,30
Commotio Cordis
Although not directly related to a cardiovascular abnormality, commotio cordis deserves mention. Literally meaning “concussion of the heart,” commotio cordis death results from a fatal dysrhythmia induced by the transfer of kinetic energy by a nonpenetrating projectile (baseball, softball, or hockey puck) striking the chest. It is thought that the blow must occur at a particularly vulnerable phase of the cardiac cycle (ventricular repolarization) to be fatal.39,40 Fortunately, episodes of commotio cordis are rare, because the rate of resuscitation is remarkably low.30
ON-FIELD RESPONSE
Any athlete who collapses on or off the field should be assessed for the presence of respirations and pulse. If these are absent, cardiopulmonary resuscitation should be initiated and the emergency medical system activated. The final common pathway in nearly all cases of SCD is a fatal ventricular arrhythmia.41 Chances for successful resuscitation are remote, even if cardiopulmonary resuscitation is started immediately and defibrillation equipment is readily available.16 With survival unlikely once an arrhythmia is triggered, the only potential opportunity to prevent such tragedies is to identify susceptible individuals before a fatal event.
PAST EXPERIENCES WITH CARDIAC SCREENING
As I have presented, numerous potential causes of SCD exist; however, only a few abnormalities claim most lives. Many of the conditions present with ominous symptoms such as exercise-related syncope, exertional dyspnea, or chest pain.7,15,16,42 In addition, some individuals with HCM may have a family history of SCD. However, two thirds of athletes with potentially fatal cardiac disease or cardiac anomalies present with SCD.7,43 Disturbingly, Maron et al7 found that of the 115 persons experiencing SCD who had undergone a preparticipation medical evaluation, only 4 (3%) were suspected of having cardiac disease. The correct diagnosis was made in only one athlete before death.
Large population studies have revealed that the standard history and physical examination result in an extremely small number of significant cardiac findings.44,45 Therefore, efforts have been made to discover potentially serious cardiovascular abnormalities before athletic participation begins. Diagnostic evaluation beyond a thorough history and physical examination may identify a small percentage of individuals at risk for SCD. A number of studies have been conducted in the United States using echocardiography and ECGs as screening instruments. In one of the larger studies to date,46 2997 athletes were screened by echocardiography; no disqualifying cardiac abnormalities were discovered. In another study,47 501 college athletes were screened with a family and personal history and an ECG. Ninety athletes (18%) then underwent an echocardiogram to rule out potential abnormalities (75 due to an abnormal ECG result). No athletes were restricted from participation. The screening ECG has been further studied and is widely believed to be ineffective because of a significant false-positive rate.47–50
Lewis et al49 performed echocardiography on 265 black athletes at Howard University, revealing 14 cases (5.3%) of mitral valve prolapse but no cases of HCM or other disqualifying pathologic condition. Murry et al,48 using screening echocardiography, found no lesions to preclude activity in the 125 athletes screened. Interestingly, one screened athlete had an episode of syncope the next day at football practice. Despite extensive testing, no diagnosis had been established before the publication of their study.
Results from Italy's long-running screening program (details discussed herein) show that such endeavors can uncover significant pathologic findings.32,51 From 1979 to 1996, 33 735 young athletes (all younger than 35 years) were screened at a regional sports medicine center in the Veneto region of Italy.51 A total of 3016 individuals (8.9%) were referred for echocardiography because of family history, abnormal physical examination findings, or ECG abnormalities. In 22 individuals (20 male and 2 female athletes), HCM was diagnosed, disqualifying them from competition. In all, 621 athletes (1.8%) were disqualified for cardiovascular conditions including rhythm and conduction abnormalities, systemic hypertension, valvular disease, and HCM.
POTENTIAL SEQUELAE OF SCREENING PROGRAMS
Although diagnostic tools such as ECGs and echocardiography may identify a small number of individuals at risk for SCD, the emotional and financial costs are high given the limits on the screening instruments currently available. With the low prevalence of cardiac anomalies, it has been estimated that 200 000 individual athletes would need to be screened to identify the single individual who would die suddenly.52 This means that even if a near-perfect screening test existed, with a specificity and sensitivity of 99%, we would be left with only 1 truly positive test result for the 1999 false-positive results generated. We can easily extrapolate the number of false-positive results that would be created nationwide, considering the millions of high school and college athletes who participate in sports each year.
Many may argue that any effort is worthwhile if it saves lives, regardless of cost. The Italian government has screened all athletes ages 12 to 35 years on an annual basis since 1971.32,51 The screening battery includes a history and physical examination, exercise and pulmonary function testing, and an ECG.16 The program is funded by the country's National Health Service. Interestingly, in the event of an incorrect diagnosis that leads directly to death or impaired health, the physician who cleared the athlete for competition may be held liable in civil and criminal court.16 Although Italian researchers report a much lower rate of SCD due to HCM,51 the overall SCD incidence of 1.6 in 100 000 young athletes in Italy is similar to the rate seen in the United States.
Screening all athletes with echocardiography would certainly identify many potential causes of SCD but at an astounding financial cost (average echocardiogram cost, $857). Even if economically possible, instituting screening nationwide would be difficult because of limited availability of testing in many rural areas. Screening by ECG is less expensive, but as discussed, the high false-positive rate would necessitate further testing in up to 10% of screened athletes.
More importantly, the emotional costs of such testing would be far greater. The large number of false-positive results generated would create worry and stress in parents and athletes as they awaited further testing. Such worry may affect later participation in competitive sports and recreational activities, with the specter of sudden death haunting the athlete. Similar effects have been described in children diagnosed as having innocent heart murmurs53 and the well-known “cardiac cripple” syndrome seen after a myocardial infarction.
Another pitfall of screening is that we are unable to stratify risk based on the severity of HCM or any other lesion discovered. Therefore, the universal recommendation is the avoidance of “intense” physical activity. Of course, the presence of HCM or other potentially fatal heart pathologic condition does not uniformly result in SCD.54,55 The diagnosis of a potentially fatal heart condition is no guarantee that a previously healthy, active athlete will suddenly and willingly adopt a sedentary lifestyle. Disqualification from sanctioned athletics based on the presence of a potentially fatal heart condition has been legally challenged.2 Recently, however, a US Court of Appeals cited the 26th Bethesda Conference56 as the guidelines physicians should rely on when formulating decisions on participation for athletes with cardiovascular disease, perhaps setting a precedent for future court cases.57 Unfortunately, even athletes who understand their risk of SCD will sometimes continue to participate in intense recreational activity, occasionally with fatal results.
CURRENT RECOMMENDATIONS FOR CARDIAC SCREENING
Recognizing the significant limitations and great costs of mass population screening of athletes for cardiovascular abnormalities, the American Heart Association (AHA) assembled a panel of experts in 1996 and developed “recommendations and guidelines for the most prudent, practical, and effective screening procedures and strategies.”9 Although not specifically discouraging the use of screening ECGs or echocardiography, the panel pointed out that, given the low prevalence of cardiac disease, the implementation of such programs on a wide scale would likely result in a greater number of false-positive than true-positive results.
The panel did recommend that all high school and college athletes undergo a cardiovascular evaluation before athletic participation, performed by a health care worker trained in the evaluation of cardiovascular disease, preferably a licensed physician. Such screening should be repeated every 2 years. The evaluation should consist of the following elements (Table 4): (1) family history of premature or sudden death or heart disease in any surviving relatives; (2) personal history of heart murmur, systemic hypertension, excessive fatigue, exertional chest pain, exertional syncope, or excessive shortness of breath; (3) physical assessment for heart murmur, femoral pulses, stigmata of Marfan syndrome (Table 3), and brachial artery blood pressure; and (4) parental verification of history form by signature for high school athletes.
Table 4.
American Heart Association Recommended Screening Items

If cardiac disease is identified through screening and referral results in a definitive cardiac diagnosis, recommendations regarding further competitive and recreational activity should be formulated in accordance with the consensus panel guidelines of the 26th Bethesda Conference.56
CURRENT STATUS OF CARDIAC SCREENING
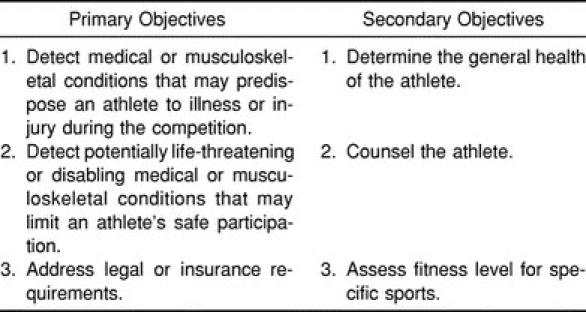
Unfortunately, many physicians, athletic trainers, and administrators continue to face the annual “sports physical” event with a certain loathing. Although there is no legal precedent for conducting such examinations, there is an implicit moral and ethical responsibility on the part of the institution (high school or college) to provide such a service.9 The objectives of the PAE have been well described (Table 5). Despite this, all involved often view the examination as an inconvenience. The manner in which most PAEs are conducted nationwide reflects this sentiment.
Table 5.
Primary and Secondary Objectives of the Preparticipation Athletic Evaluation
The preparticipation cardiovascular screening process at the high school and college level in the United States is currently not meeting the standards set by the AHA.58–60 This is despite the increased focus on SCD during the past decade by many in the sports medicine community and numerous publications,10,13–15,42 including a well-publicized monograph,12 which have highlighted the need for improvement. When compared with the AHA guidelines, only 26% of National Collegiate Athletic Association (NCAA) schools were found to be using “adequate” history and physical examination forms (containing at least 9 of 12 recommended items).58 NCAA Division II and Division III schools (28% and 30%, respectively) were found to have inadequate forms (containing 4 or fewer of the 12 recommended items) more often than Division I schools (14%). Interestingly, 75% of NCAA team physicians were orthopaedic surgeons, physicians with little or no postgraduate training in the evaluation of cardiac disease.
The status of examinations in high schools nationwide is predictably even more dismal. In the absence of national standardization of PAE forms, only 17 of 43 state forms recently evaluated were found to be “adequate” with regard to AHA recommendations.60 Gomez et al59 surveyed a sample of certified athletic trainers at high schools nationwide and found that only 17.2% of returned PAE forms contained all elements of the cardiovascular history recommended by Lombardo et al.11 In Oregon, the state athletic association has only recently recommended a form for use by member schools.61 In a survey conducted by our institution (unpublished data, 2000), we found that a large number of schools continued to use suboptimal forms despite the recommendation.
Reviewing the forms used during the PAE process gives insight into what history is obtained and the scope of the physical examination performed. Such guidelines are important, since the practitioners performing PAEs nationwide vary markedly. Five states have no specific recommendations, requirements, or restrictions with regard to who may perform the PAE.60 Of the remaining 46 jurisdictions (including the District of Columbia), 21 allow physician assistants and nurse practitioners to perform examinations, whereas chiropractors are approved to do so in 10 states and naturopathic clinicians in a single jurisdiction. No state offers specific qualifications for examiners or guidelines for the examinations. Although the cardiovascular system training and experience of physician assistants and nurse practitioners may vary greatly, chiropractors and naturopaths have limited training in the detection of cardiovascular disease.60
When examinations are conducted in accordance with AHA recommendations by appropriately trained practitioners, potentially serious cardiac pathologic conditions may be detected. As previously discussed, few athletes have symptoms or physical examination findings before SCD, and the current method of screening has a very low yield. However, because almost all institutions currently “go through the motions” of the PAE on a yearly basis, most need to make only minimal changes (use of appropriate forms and properly trained examiners) to improve the ability to detect many clinically significant cardiac conditions in the young athlete.
CONCLUSIONS
Fortunately, most athletic trainers will never experience the sudden death of an athlete, because it is an extremely rare event. Despite the variety of causes of SCD, only a few conditions are responsible for most deaths. Most athletes who eventually succumb to SCD have no history of cardiac problems (family or personal) and no symptoms before death. Hence, the detection of at-risk individuals poses a significant challenge to the sports medicine team. Population screening by diagnostic testing is not currently economically or practically feasible, but a significant proportion of at-risk athletes can be identified through a thorough history and physical examination.
Although no perfect screening instrument is currently available, a moral and ethical obligation exists for physicians and athletic trainers to ensure that athletes are assessed in the most prudent and efficient manner available. Therefore, the recommendations of the AHA should be considered the minimum standard for the cardiovascular screening of high school and college athletes nationwide. Current data show that we are doing a woeful job of implementing these standards. Athletic trainers are often responsible for the organization and administration of the PAE and must work with their team physicians to implement such changes. In addition, all athletic trainers should offer their expertise to administrators at high schools without sports medicine services. As physicians and athletic trainers, we recognize that athletic participation carries inherent risks. However, when we are capable of minimizing those risks, we must have the professional integrity to act within our capacity to do so.
ACKNOWLEDGMENTS
Presented in part at the Oregon Athletic Trainers' Society Summer Symposium, June 24, 2000, Bend, OR.
REFERENCES
- 1.Maron BJ. Sudden death in young athletes: lessons from the Hank Gathers affair. N Engl J Med. 1993;329:55–57. doi: 10.1056/NEJM199307013290113. [DOI] [PubMed] [Google Scholar]
- 2.Mitten MJ, Maron BJ. Legal considerations that affect medical eligibility for competitive athletes with cardiovascular abnormalities and acceptance of Bethesda Conference recommendations. J Am Coll Cardiol. 1994;24:861–863. doi: 10.1016/0735-1097(94)90840-0. [DOI] [PubMed] [Google Scholar]
- 3.Hudson MA. A legacy on court, in court. Los Angeles Times. 1992 Oct 6;:A1. [Google Scholar]
- 4.Fainaru S, Foreman J, Golden D, MacMullan J, Manly H, Kurkjian S. The death of Reggie Lewis: a search for answers. Boston Globe. 1993 Sep 12;:70–76. [Google Scholar]
- 5.Van Camp S. What can we learn from Reggie Lewis' death? Physician Sportsmed. 1993;21(10):73–87. doi: 10.1080/00913847.1993.11710431. [DOI] [PubMed] [Google Scholar]
- 6.Thompson PD. Athletes, athletics, and sudden cardiac death. Med Sci Sports Exerc. 1993;25:981–984. [PubMed] [Google Scholar]
- 7.Maron BJ, Shirani J, Poliac LC, Mathenge R, Roberts WC, Mueller FO. Sudden death in young competitive athletes: clinical, demographic, and pathological profiles. JAMA. 1996;276:199–204. [PubMed] [Google Scholar]
- 8.Van Camp SP, Bloor CM, Mueller FO, Cantu RC, Olson HG. Nontraumatic sports death in high school and college athletes. Med Sci Sports Exerc. 1995;27:641–647. [PubMed] [Google Scholar]
- 9.Maron MJ, Thompson PD, Puffer JC, et al. Cardiovascular preparticipation screening of competitive athletes: a statement for health professionals from the Sudden Death Committee and Congenital Cardiac Defects Committee (cardiovascular disease in the young), American Heart Association. Circulation. 1996;94:850–856. doi: 10.1161/01.cir.94.4.850. [DOI] [PubMed] [Google Scholar]
- 10.Koester MC. Refocusing the adolescent preparticipation physical evaluation toward preventive health care. J Athl Train. 1995;30:352–360. [PMC free article] [PubMed] [Google Scholar]
- 11.Lombardo JA, Robinson JB, Smith DM. Preparticipation Physical Evaluation. Kansas City, MO: American Academy of Family Physicians, American Academy of Pediatricians, American Medical Society for Sports Medicine, American Orthopedic Society for Sports Medicine, American Osteopathic Academy of Sports Medicine; 1992. [Google Scholar]
- 12.Smith DM, Kovan JR, Rich BSE, Tanner SM. Preparticipation Physical Evaluation. 2nd ed. Minneapolis, MN: McGraw-Hill Inc; 1997. [Google Scholar]
- 13.Hergenroeder AC. The preparticipation sports examination. Pediatr Clin North Am. 1997;44:1525–1540. doi: 10.1016/s0031-3955(05)70572-1. [DOI] [PubMed] [Google Scholar]
- 14.Smith DM. Pre-participation physical evaluations: development of uniform guidelines. Sports Med. 1994;18:293–300. doi: 10.2165/00007256-199418050-00001. [DOI] [PubMed] [Google Scholar]
- 15.Rich BS. Sudden death screening. Med Clin North Am. 1994;78:267–288. doi: 10.1016/s0025-7125(16)30159-6. [DOI] [PubMed] [Google Scholar]
- 16.Maron BJ. Cardiovascular risks to young persons on the athletic field. Ann Intern Med. 1998;129:379–386. doi: 10.7326/0003-4819-129-5-199809010-00006. [DOI] [PubMed] [Google Scholar]
- 17.Myerburg RJ. Sudden death. J Continuing Educ Cardiol. 1978;13:15–29. [Google Scholar]
- 18.Van Camp SP. Sudden death. Clin Sports Med. 1992;11:273–289. [PubMed] [Google Scholar]
- 19.Phillips M, Robinowitz M, Higgins JR, Boran KJ, Reed T, Virmani R. Sudden cardiac death in Air Force recruits: a 20-year review. JAMA. 1986;256:2696–2699. [PubMed] [Google Scholar]
- 20.Maron BJ, Gohman TE, Aeppli D. Prevalence of sudden cardiac death during competitive sports activities in Minnesota high school athletes. J Am Coll Cardiol. 1998;32:1881–1884. doi: 10.1016/s0735-1097(98)00491-4. [DOI] [PubMed] [Google Scholar]
- 21.Ragosta M, Crabtree J, Sturner WQ, Thompson PD. Death during recreational exercise in the State of Rhode Island. Med Sci Sports Exerc. 1984;16:339–342. [PubMed] [Google Scholar]
- 22.Futterman LG, Myerburg R. Sudden death in athletes: an update. Sports Med. 1998;26:335–350. doi: 10.2165/00007256-199826050-00004. [DOI] [PubMed] [Google Scholar]
- 23.Wight JN, Jr, Salem D. Sudden cardiac death and the “athlete's heart.”. Arch Intern Med. 1995;155:1473–1480. [PubMed] [Google Scholar]
- 24.Maron BJ, Pelliccia A, Spirito P. Cardiac disease in young trained athletes: insights into methods for distinguishing athlete's heart from structural heart disease, with particular emphasis on hypertrophic cardiomyopathy. Circulation. 1995;91:1596–1601. doi: 10.1161/01.cir.91.5.1596. [DOI] [PubMed] [Google Scholar]
- 25.McCaffrey FM, Braden DS, Strong WB. Sudden cardiac death in young athletes: a review. Am J Dis Child. 1991;145:177–183. doi: 10.1001/archpedi.1991.02160020069020. [DOI] [PubMed] [Google Scholar]
- 26.Maron BJ, Roberts WC, Epstein SE. Sudden death in hypertrophic cardiomyopathy: a profile of 78 patients. Circulation. 1982;65:1388–1394. doi: 10.1161/01.cir.65.7.1388. [DOI] [PubMed] [Google Scholar]
- 27.Maron BJ, Roberts WC, McAllister HA, Rosing DR, Epstein SE. Sudden death in young athletes. Circulation. 1980;62:218–229. doi: 10.1161/01.cir.62.2.218. [DOI] [PubMed] [Google Scholar]
- 28.Maron BJ, Gardin JM, Flack JM, Gidding SS, Kurosaki TT, Bild D. Prevalence of hypertrophic cardiomyopathy in a general population of young adults: echocardiographic analysis of 4111 subjects in CARDIA Study. Coronary Artery Risk Development in (Young) Adults. Circulation. 1995;92:785–789. doi: 10.1161/01.cir.92.4.785. [DOI] [PubMed] [Google Scholar]
- 29.Maron BJ, Roberts WC. Quantitative analysis of cardiac muscle cell disorganization in the ventricular septum of patients with hypertrophic cardiomyopathy. Circulation. 1979;59:689–706. doi: 10.1161/01.cir.59.4.689. [DOI] [PubMed] [Google Scholar]
- 30.Reisdorff EJ, Prodinger RJ. Sudden cardiac death in the athlete. Emerg Med Clin North Am. 1998;16:281–294. doi: 10.1016/s0733-8627(05)70004-3. [DOI] [PubMed] [Google Scholar]
- 31.Cheitlin MD, De Castro CM, McAllister HA. Sudden death as a complication of anomalous left coronary origin from the anterior sinus of Valsalva: a not-so-minor congenital anomaly. Circulation. 1974;50:780–787. doi: 10.1161/01.cir.50.4.780. [DOI] [PubMed] [Google Scholar]
- 32.Thiene G, Basso C, Corrado D. Is prevention of sudden death in young athletes feasible? Cardiologia. 1999;44:497–505. [PubMed] [Google Scholar]
- 33.Liberthson RR, Dinsmore RE, Fallon JT. Aberrant coronary artery origin from the aorta: report of 18 patients, review of literature and delineation of natural history and management. Circulation. 1979;59:748–754. doi: 10.1161/01.cir.59.4.748. [DOI] [PubMed] [Google Scholar]
- 34.Basso C, Maron BJ, Corrado D, Thiene G. Clinical profile of congenital coronary artery anomalies with origin from the wrong aortic sinus leading to sudden death in young competitive athletes. J Am Coll Cardiol. 2000;35:1493–1501. doi: 10.1016/s0735-1097(00)00566-0. [DOI] [PubMed] [Google Scholar]
- 35.Bresler MJ. Acute pericarditis and myocarditis. Emerg Med. 1992;24:35–51. [Google Scholar]
- 36.Lecomte D, Fornes P, Fouret P, Nicholas G. Isolated myocardial fibrosis as a cause of sudden cardiac death and its possible relation to myocarditis. J Forensic Sci. 1993;38:617–621. [PubMed] [Google Scholar]
- 37.McKeag DB. Preparticipation screening of the potential athlete. Clin Sports Med. 1989;8:373–397. [PubMed] [Google Scholar]
- 38.Ackerman MJ. The long QT syndrome. Pediatr Rev. 1998;19:232–238. doi: 10.1542/pir.19-7-232. [DOI] [PubMed] [Google Scholar]
- 39.Estes NA., III Sudden death in young athletes. N Engl J Med. 1995;333:337–341. doi: 10.1056/NEJM199508103330611. [DOI] [PubMed] [Google Scholar]
- 40.Maron BJ, Poliac LC, Kaplan JA, Mueller FO. Blunt impact to the chest leading to sudden death from cardiac arrest during sports activities. N Engl J Med. 1995;333:337–342. doi: 10.1056/NEJM199508103330602. [DOI] [PubMed] [Google Scholar]
- 41.Furlanello F, Bettini R, Cozzi F, et al. Ventricular arrhythmias and sudden death in athletes. Ann N Y Acad Sci. 1984;427:253–279. doi: 10.1111/j.1749-6632.1984.tb20789.x. [DOI] [PubMed] [Google Scholar]
- 42.O'Connor FG, Kugler JP, Oriscello RG. Sudden death in young athletes: screening for the needle in the haystack. Am Fam Physician. 1998;57:2763–2770. [PubMed] [Google Scholar]
- 43.Zehender M, Meinertz T, Keul J, Just H. ECG variants and cardiac arrhythmias in athletes: clinical relevance and prognostic importance. Am Heart J. 1990;119:1378–1391. doi: 10.1016/s0002-8703(05)80189-9. [DOI] [PubMed] [Google Scholar]
- 44.Magnes SA, Henderson JM, Hunter SC. What conditions limit sports participation? Experience with 10,540 athletes. Physician Sportsmed. 1992;20(5):143–160. doi: 10.1080/00913847.1992.11947434. [DOI] [PubMed] [Google Scholar]
- 45.Smith J, Laskowski ER. The preparticipation physical examination: Mayo Clinic experience with 2,739 examinations. Mayo Clin Proc. 1998;73:419–429. doi: 10.1016/S0025-6196(11)63723-3. [DOI] [PubMed] [Google Scholar]
- 46.Weidenbener EJ, Krauss MD, Waller BF, Taliercio CP. Incorporation of screening echocardiography in the preparticipation exam. Clin J Sport Med. 1995;5:86–89. doi: 10.1097/00042752-199504000-00003. [DOI] [PubMed] [Google Scholar]
- 47.Maron BJ, Bodison SA, Wesley YE, Tucker E, Green KJ. Results of screening a large group of intercollegiate competitive athletes for cardiovascular disease. J Am Coll Cardiol. 1987;10:1214–1221. doi: 10.1016/s0735-1097(87)80121-3. [DOI] [PubMed] [Google Scholar]
- 48.Murry PM, Cantwell JD, Heath DL, Shoop J. The role of limited echocardiography in screening athletes. Am J Cardiol. 1995;76:849–850. doi: 10.1016/s0002-9149(99)80244-6. [DOI] [PubMed] [Google Scholar]
- 49.Lewis JF, Maron BJ, Diggs JA, Spencer JE, Mehrotra PP, Curry CL. Preparticipation echocardiographic screening for cardiovascular disease in a large, predominantly black population of collegiate athletes. Am J Cardiol. 1989;64:1029–1033. doi: 10.1016/0002-9149(89)90802-3. [DOI] [PubMed] [Google Scholar]
- 50.Fuller CM, McNulty CM, Spring DA, et al. Prospective screening of 5,615 high school athletes for risk of sudden cardiac death. Med Sci Sports Exerc. 1997;29:1131–1138. doi: 10.1097/00005768-199709000-00003. [DOI] [PubMed] [Google Scholar]
- 51.Corrado D, Basso C, Schiavon M, Thiene G. Screening for hypertrophic cardiomyopathy in young athletes. N Engl J Med. 1998;339:364–369. doi: 10.1056/NEJM199808063390602. [DOI] [PubMed] [Google Scholar]
- 52.Ades PA. Preventing sudden death. Physician Sportsmed. 1992;20(9):75–89. doi: 10.1080/00913847.1992.11947484. [DOI] [PubMed] [Google Scholar]
- 53.Bergman AB, Stamm SJ. The morbidity of cardiac nondisease in schoolchildren. N Engl J Med. 1967;276:1008–1013. doi: 10.1056/NEJM196705042761804. [DOI] [PubMed] [Google Scholar]
- 54.Maron BJ, Wesley YE, Arce J. Hypertrophic cardiomyopathy compatible with successful completion of the marathon. Am J Cardiol. 1984;53:1470–1471. doi: 10.1016/s0002-9149(84)91437-1. [DOI] [PubMed] [Google Scholar]
- 55.Simons SM, Moriarity J. Hypertrophic cardiomyopathy in a college athlete. Med Sci Sports Exerc. 1992;24:1321–1324. [PubMed] [Google Scholar]
- 56.Maron BJ, Isner JM, McKenna WJ. 26th Bethesda Conference: recommendations for determining eligibility for competition in athletes with cardiovascular abnormalities. Task Force 3: hypertrophic cardiomyopathy, myocarditis and other myopericardial diseases and mitral valve prolapse. J Am Coll Cardiol. 1994;24:880–885. doi: 10.1016/0735-1097(94)90844-3. [DOI] [PubMed] [Google Scholar]
- 57.Knapp v Northwestern University. 101F 3d 473 (7th Cir 1996)
- 58.Pfister GC, Puffer JC, Maron BJ. Preparticipation cardiovascular screening for US collegiate student-athletes. JAMA. 2000;283:1597–1599. doi: 10.1001/jama.283.12.1597. [DOI] [PubMed] [Google Scholar]
- 59.Gomez JE, Lantry BR, Saathoff KNS. Current use of adequate preparticipation history forms for heart disease screening of high school athletes. Arch Pediatr Adolesc Med. 1999;153:723–726. doi: 10.1001/archpedi.153.7.723. [DOI] [PubMed] [Google Scholar]
- 60.Glover DW, Maron BJ. Profile of preparticipation cardiovascular screening for high school athletes. JAMA. 1998;279:1817–1819. doi: 10.1001/jama.279.22.1817. [DOI] [PubMed] [Google Scholar]
- 61.Oregon Schools Activities Association. Minutes of spring 1999 meeting. Available at: http://www.osaa.org/events/meet#May3,1999. Accessed May 5, 2000.