Abstract
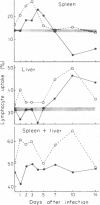
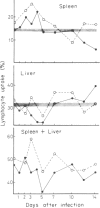
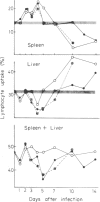
Normal lymphocytes labelled with 51Cr were injected into mice at various stages of lethal and non-lethal malaria infections. Marked alterations were seen in the uptake into spleen and liver, which correlated with the outcome of the infection. Non-lethal infections and lethal infections in mice protected by vaccination caused increased uptakes, especially in the liver. In lethal infections, particularly Plasmodium berghei, uptakes were below normal values at certain times: this was apparently due to destruction of lymphocytes, probably caused by autoantibody.
Full text
PDF
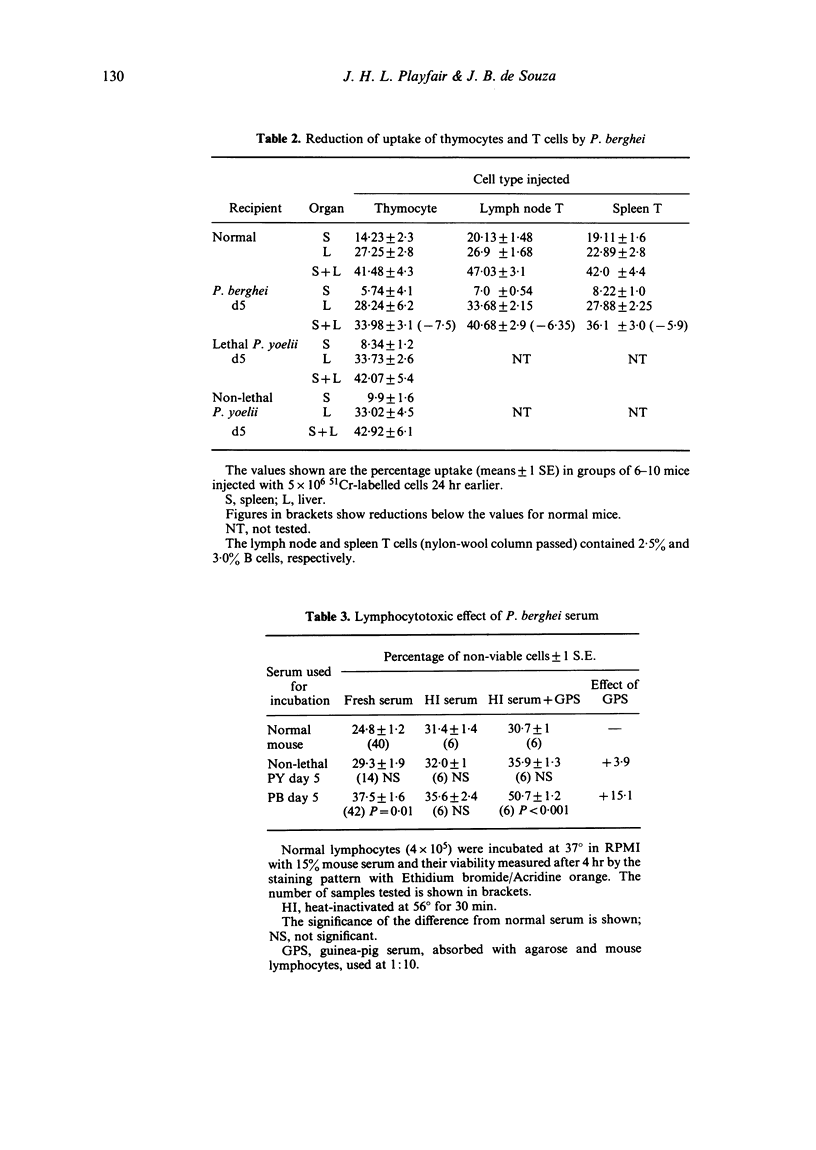



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brissette W. H., Coleman R. M., Rencricca N. J. Depressed splenic T lymphocyte numbers and thymocyte migratory patterns in murine malaria. Proc Soc Exp Biol Med. 1978 Nov;159(2):317–320. doi: 10.3181/00379727-159-40340. [DOI] [PubMed] [Google Scholar]
- Bullock W. E., Jr Perturbation of lymphocyte circulation in experimental murine leprosy. II. Nature of the defect. J Immunol. 1976 Oct;117(4):1171–1178. [PubMed] [Google Scholar]
- Chung P. R., Balsamo E., Gray A., Rencricca N. J., Coleman R. M. Autocytotoxins during rodent malaria. J Parasitol. 1980 Oct;66(5):847–849. [PubMed] [Google Scholar]
- Cottrell B. J., Playfair J. H., De Souza B. J. Cell-mediated immunity in mice vaccinated against malaria. Clin Exp Immunol. 1978 Nov;34(2):147–158. [PMC free article] [PubMed] [Google Scholar]
- Dockrell H. M., de Souza J. B., Playfair J. H. The role of the liver in immunity to blood-stage murine malaria. Immunology. 1980 Oct;41(2):421–430. [PMC free article] [PubMed] [Google Scholar]
- Hutchinson I. V. Antigen-reactive cell opsonization (ARCO) and its role in antibody-mediated immune suppression. Immunol Rev. 1980;49:167–197. doi: 10.1111/j.1600-065x.1980.tb00430.x. [DOI] [PubMed] [Google Scholar]
- Lelchuk R., Playfair J. H. Two distinct types of non-specific immunosuppression in murine malaria. Clin Exp Immunol. 1980 Dec;42(3):428–435. [PMC free article] [PubMed] [Google Scholar]
- Lelchuk R., Sprott V. M., Playfair J. H. Differential involvement of non-specific suppressor T cells in two lethal murine malaria infections. Clin Exp Immunol. 1981 Aug;45(2):433–438. [PMC free article] [PubMed] [Google Scholar]
- Playfair J. H., De Souza J. B. Antibody responses in mice protected against malaria by vaccination. Parasite Immunol. 1979 Autumn;1(3):197–208. doi: 10.1111/j.1365-3024.1979.tb00706.x. [DOI] [PubMed] [Google Scholar]
- Playfair J. H., De Souza J. B., Cottrell B. J. Protection of mice against malaria by a killed vaccine: differences in effectiveness against P. yoelii and P. berghei. Immunology. 1977 Oct;33(4):507–515. [PMC free article] [PubMed] [Google Scholar]
- Playfair J. H., De Souza J. B., Dockrell H. M., Agomo P. U., Taverne J. Cell-mediated immunity in the liver of mice vaccinated against malaria. Nature. 1979 Dec 13;282(5740):731–734. doi: 10.1038/282731a0. [DOI] [PubMed] [Google Scholar]
- Wells R. A., Pavanand K., Zolyomi S., Permpanich B., MacDermott R. P. Loss of circulating T lymphocytes with normal levels of B and 'null' lymphocytes in Thai adults with malaria. Clin Exp Immunol. 1979 Feb;35(2):202–209. [PMC free article] [PubMed] [Google Scholar]
- Wyler D. J. Peripheral lymphocyte subpopulations in human falciparum malaria. Clin Exp Immunol. 1976 Mar;23(3):471–476. [PMC free article] [PubMed] [Google Scholar]