Abstract
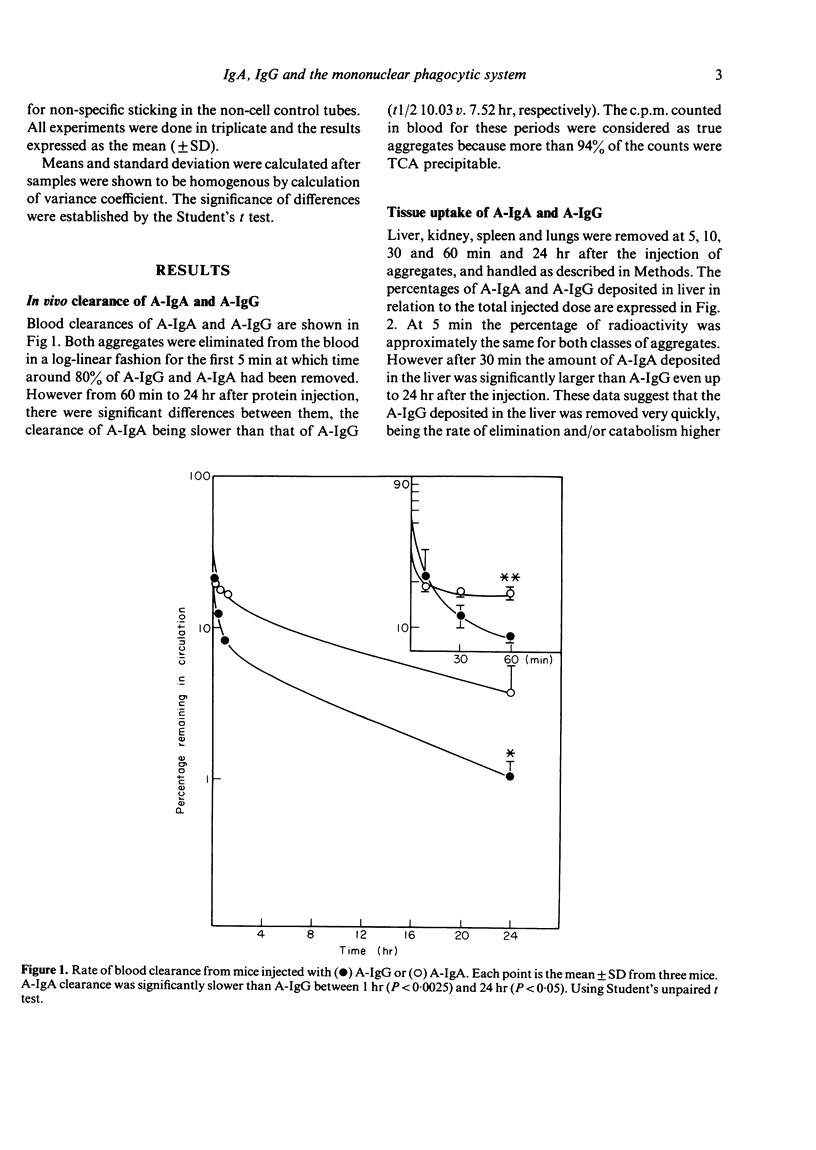
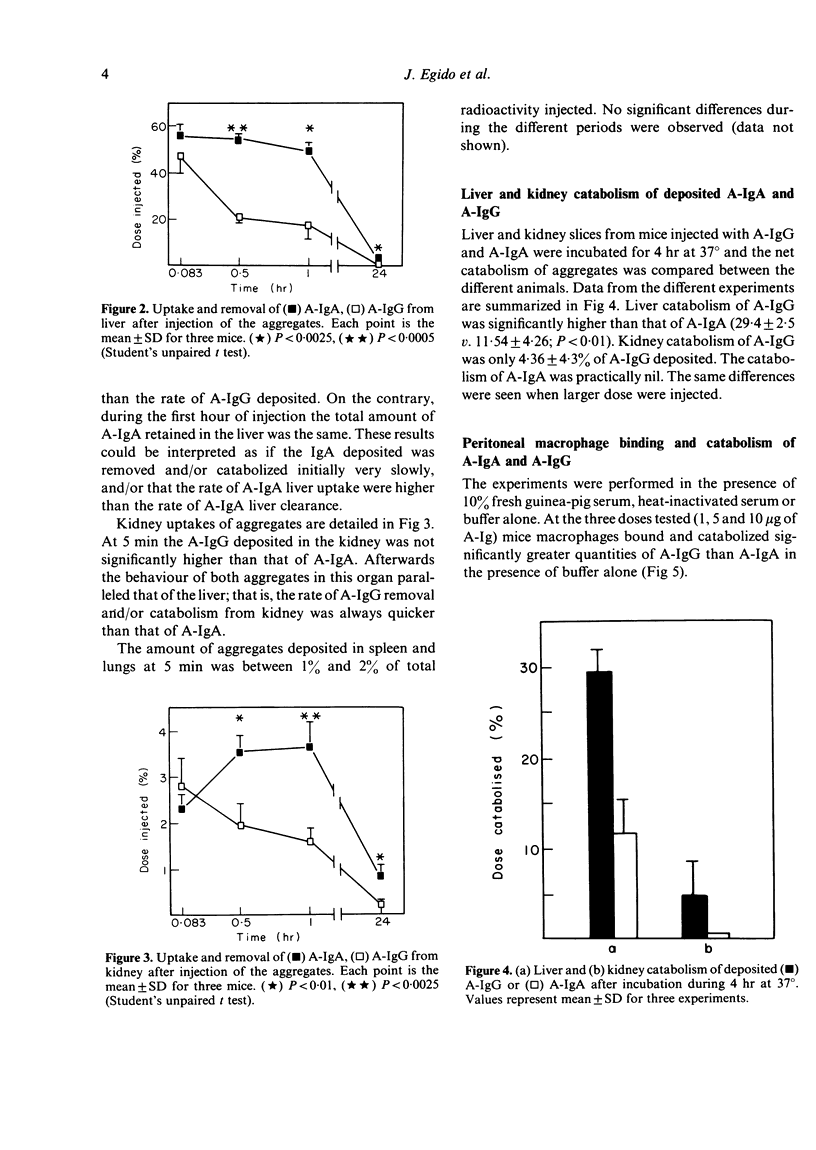
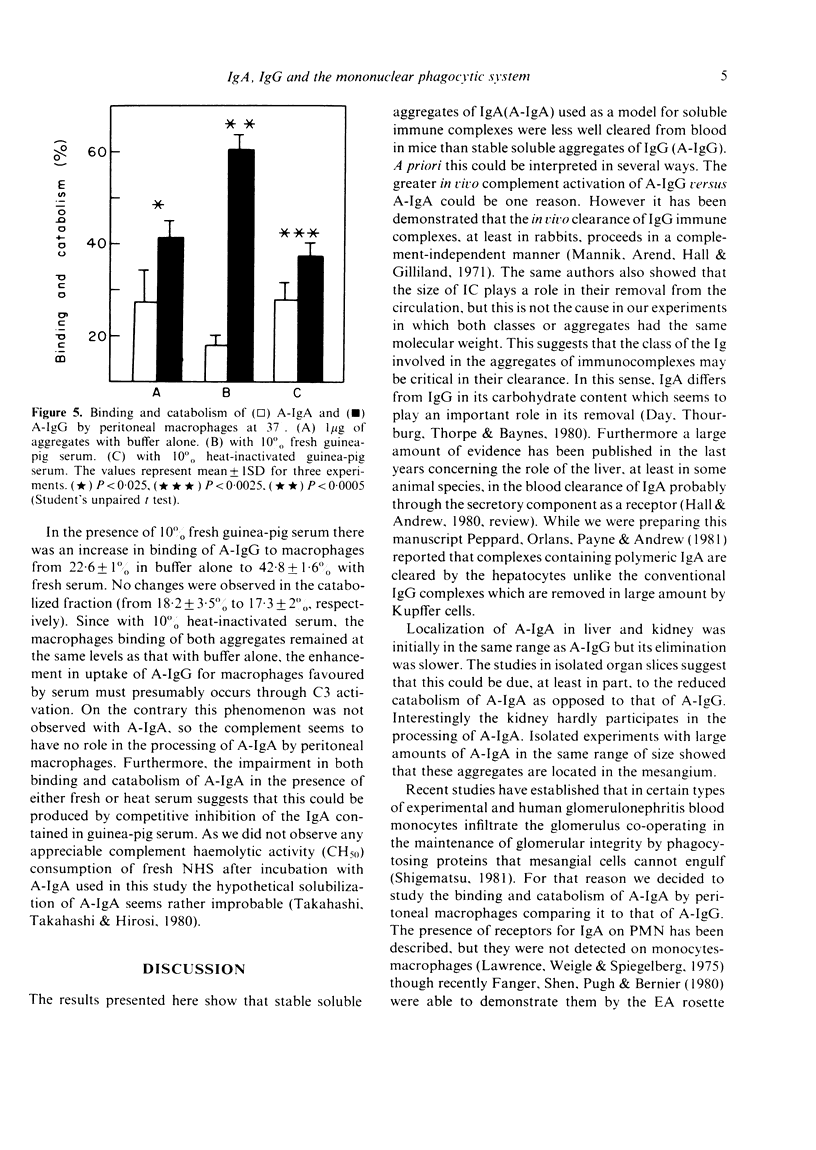
We have studied the fate of soluble stable aggregates of human IgA (A-IgA) and IgG (A-IgG) after their injections in mice, as well as in vitro catabolism by liver, kidney and peritoneal macrophages. The half-life of the fast component of blood clearance was similar for both aggregates. However the half-life of the slow component of A-IgA clearance was significantly slower than A-IgG (t1/2 10 . 03 v. 7 . 52 hr, respectively). The A-IgA deposited in liver and kidney was removed significantly more slowly than A-IgG. Studies in isolated liver and kidney slices suggest that this could be due to the impaired catabolism of A-IgA as opposed to that of A-IgG. Interestingly the kidney hardly participates in the processing of A-IgA. At the three doses employed (1, 5 and 10 micrograms of both aggregates) peritoneal macrophages bound and catabolised significantly less amount of A-IgA than A-IgG. Complement seems to have no role in the processing of A-IgA by peritoneal macrophages unlike that observed with A-IgG. It is suggested that the impairment in handling of A-IgA by the mononuclear phagocytic system could provoke their persistence in the circulation and deposition at sites susceptible to injury. These results may be of some relevance for the understanding of physiological IgA-IC (immune complex) clearance and for the pathogenesis of IgA-related diseases.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Day J. F., Thornburg R. W., Thorpe S. R., Baynes J. W. Carbohydrate-mediated clearance of antibody . antigen complexes from the circulation. The role of high mannose oligosaccharides in the hepatic uptake of IgM . antigen complexes. J Biol Chem. 1980 Mar 25;255(6):2360–2365. [PubMed] [Google Scholar]
- Egido J., Sancho J., Mampaso F., Lopez Trascasa M., Sanchez Crespo M., Blasco R., Hernando L. A possible common pathogenesis of the mesangial IgA glomerulonephritis in patients with Berger's disease and Schönlein-Henoch syndrome. Proc Eur Dial Transplant Assoc. 1980;17:660–666. [PubMed] [Google Scholar]
- Fanger M. W., Shen L., Pugh J., Bernier G. M. Subpopulations of human peripheral granulocyes and monocytes express receptors for IgA. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3640–3644. doi: 10.1073/pnas.77.6.3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götze O., Müller-Eberhard H. J. The alternative pathway of complement activation. Adv Immunol. 1976;24:1–35. doi: 10.1016/s0065-2776(08)60328-4. [DOI] [PubMed] [Google Scholar]
- Harkiss G. D., Brown D. L. Clearance kinetics of soluble pre-formed immune complexes containing IgM antibodies in normal and decomplemented rabbits. Immunology. 1981 Feb;42(2):297–306. [PMC free article] [PubMed] [Google Scholar]
- Katz S. I., Hall R. P., 3rd, Lawley T. J., Strober W. Dermatitis herpetiformis: the skin and the gut. Ann Intern Med. 1980 Dec;93(6):857–874. doi: 10.7326/0003-4819-93-6-857. [DOI] [PubMed] [Google Scholar]
- Kijlstra A., Van Der Lelij A., Knutson W., Fleuren G. J., Vanes L. A. The influence of phagocyte function on glomerular localization of aggregated IgM in rats. Clin Exp Immunol. 1978 May;32(2):207–217. [PMC free article] [PubMed] [Google Scholar]
- Kijlstra A., Van Es L. A., Daha M. R. Enhanced degradation of soluble immunoglobulin aggregates by macrophages in the presence of complement. Immunology. 1979 Jul;37(3):673–680. [PMC free article] [PubMed] [Google Scholar]
- Knutson D. W., Kijlstra A., Lentz H., van Es L. A. Isolation of stable aggregates of IgG by zonal ultracentrifugation in sucrose gradients containing albumin. Immunol Commun. 1979;8(3):337–345. doi: 10.3109/08820137909050047. [DOI] [PubMed] [Google Scholar]
- Lawrence D. A., Weigle W. O., Spiegelberg H. L. Immunoglobulins cytophilic for human lymphocytes, monocytes, and neutrophils. J Clin Invest. 1975 Feb;55(2):368–376. doi: 10.1172/JCI107940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannik M., Arend M. P., Hall A. P., Gilliland B. C. Studies on antigen-antibody complexes. I. Elimination of soluble complexes from rabbit circulation. J Exp Med. 1971 Apr 1;133(4):713–739. doi: 10.1084/jem.133.4.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannik M. Physicochemical and functional relationships of immune complexes. J Invest Dermatol. 1980 May;74(5):333–338. doi: 10.1111/1523-1747.ep12543582. [DOI] [PubMed] [Google Scholar]
- McConahey P. J., Dixon F. J. A method of trace iodination of proteins for immunologic studies. Int Arch Allergy Appl Immunol. 1966;29(2):185–189. doi: 10.1159/000229699. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Takahashi S., Hirose S. Solubilization of antigen-antibody complexes: a new function of complement as a regulator of immune reactions. Prog Allergy. 1980;27:134–166. [PubMed] [Google Scholar]
- Trascasa M. L., Egido J., Sancho J., Hernando L. IgA glomerulonephritis (Berger's disease): evidence of high serum levels of polymeric IgA. Clin Exp Immunol. 1980 Nov;42(2):247–254. [PMC free article] [PubMed] [Google Scholar]
- van Snick J. L., Masson P. L. The effect of complement on the ingestion of soluble antigen-antibody complexes and IgM aggregates by mouse peritoneal macrophages. J Exp Med. 1978 Oct 1;148(4):903–914. doi: 10.1084/jem.148.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]


