Abstract
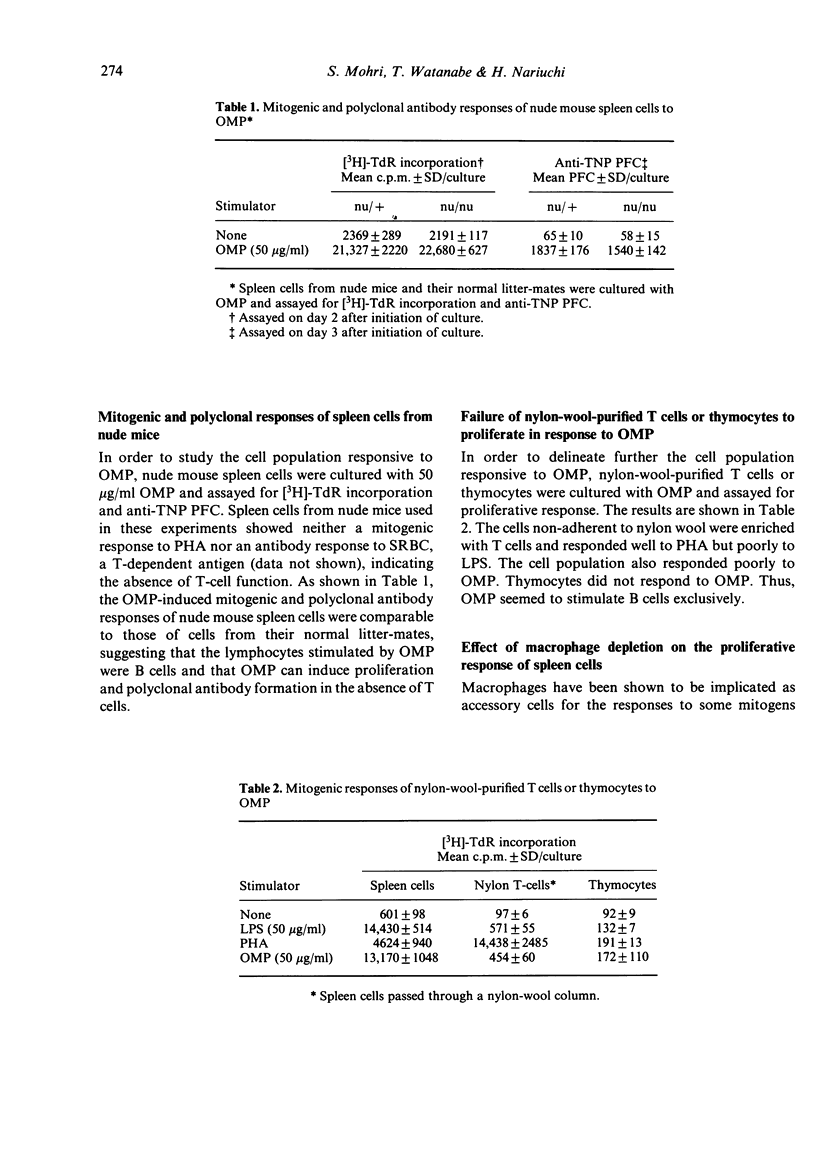
The outer membrane protein (OMP) prepared from Escherichia coli was found to be a potent mitogen for murine B cells and to be capable of inducing polyclonal antibody formation as well as a proliferative response. Spleen cells from nude mice responded equally as well to OMP as those from their normal litter-mates, whereas nylon-wool-purified T cells or thymocytes failed to respond. The proliferative response was dependent on the presence of macrophages. The macrophage dependency of the polyclonal antibody response seemed to be less than that of the proliferation. OMP was mitogenic for lipopolysaccharide (LPS)-resistant C3H/HeJ spleen cells, further indicating that OMP is an unique B-cell mitogen distinct from LPS.
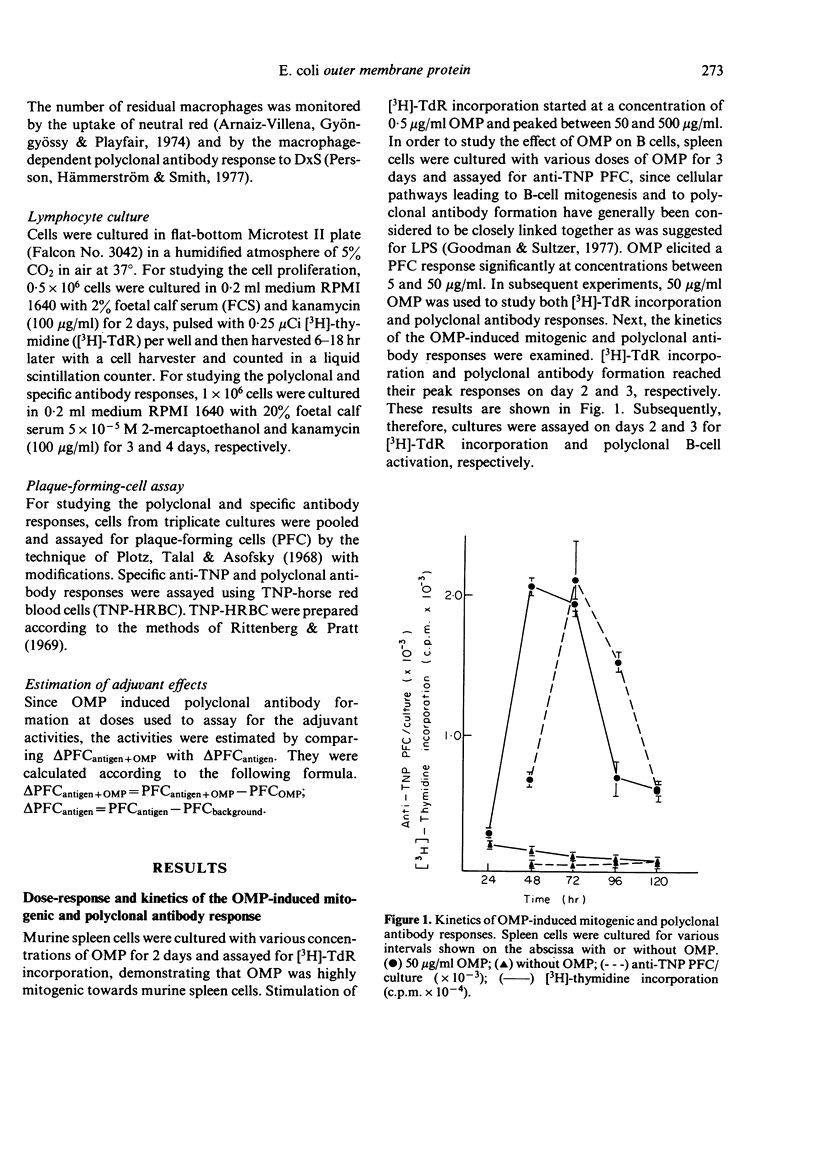
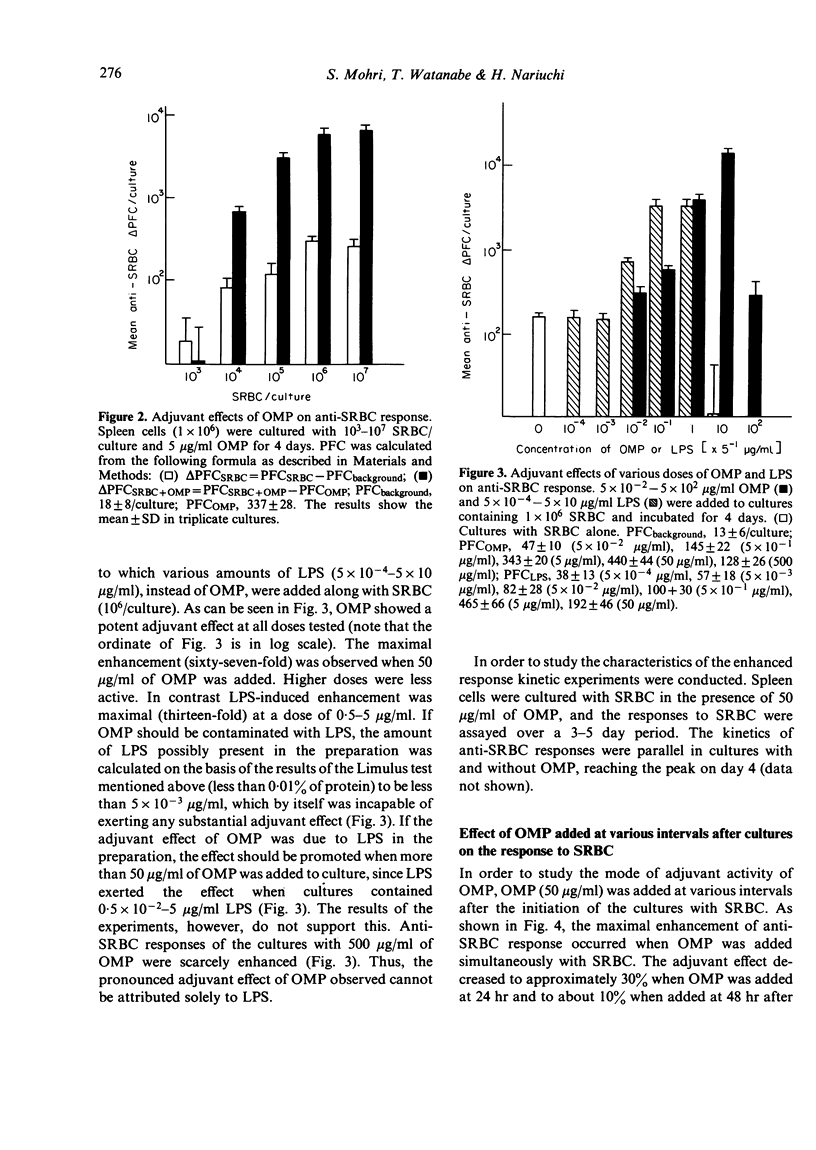
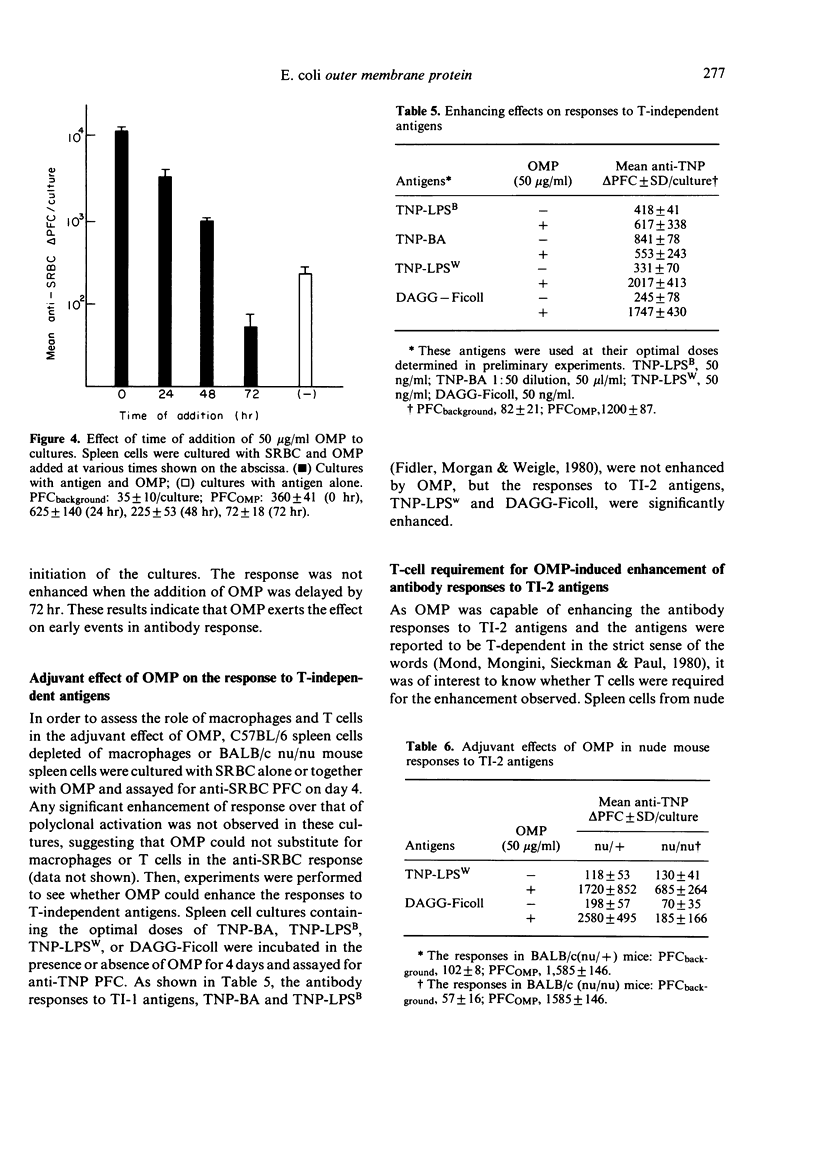
OMP also enhanced the specific antibody response sixty-seven-fold to an optimal dose of sheep red blood cells (SRBC) in vitro. The kinetics of the response, however, was not altered from that of cultures without OMP. The anti-SRBC response of spleen cells from C3H/HeJ mice was also enhanced by the addition of OMP, suggesting that the adjuvant effects were not due to the LPS in the preparation. Antibody responses in vitro to TI-1 antigens, trinitrophenyl-LPS (Boivin) (TNP-LPSB) and TNP-Brucella abortus, were not enhanced in the presence of OMP. In contrast OMP enhanced the response to TI-2 antigens, TNP-LPSW (Westphal) and dinitrophenyl-Ficoll and T cells were shown to be required for these augmented antibody responses. Enhancement was not seen in nude mouse spleen cell cultures but was seen when nylon-wool-purified T cells were added to the cultures.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnaiz-Villena A., Gyöngyössy M. I., Playfair J. H. Rosette formation by mouse lymphocytes. II. T-cell specificities in a CRL subpopulation. Clin Exp Immunol. 1974 Oct;18(2):177–186. [PMC free article] [PubMed] [Google Scholar]
- Bessler W. G., Henning U. Protein I and protein II from the outer membrane of Escherichia coli are mouse B-lymphocyte mitogens. Z Immunitatsforsch Immunobiol. 1979 Jun;155(5):387–398. [PubMed] [Google Scholar]
- Bessler W. G., Ottenbreit B. P. Studies on the mitogenic principle of the lipoprotein from the outer membrane of Escherichia coli. Biochem Biophys Res Commun. 1976 May 23;76(2):239–246. doi: 10.1016/0006-291x(77)90717-3. [DOI] [PubMed] [Google Scholar]
- DiRienzo J. M., Nakamura K., Inouye M. The outer membrane proteins of Gram-negative bacteria: biosynthesis, assembly, and functions. Annu Rev Biochem. 1978;47:481–532. doi: 10.1146/annurev.bi.47.070178.002405. [DOI] [PubMed] [Google Scholar]
- Fidler J. M., Morgan E. L., Weigle W. O. B lymphocyte differentiation in the CBA/N mouse: a delay in maturation rather than a total arrest. J Immunol. 1980 Jan;124(1):13–19. [PubMed] [Google Scholar]
- Finkelham F. D., Woods V. L., Wilburn S. B., Mond J. J., Stein K. E., Berning A., Scher I. Augmentation of in vitro humoral immune responses in the mouse by an antibody to IgD. J Exp Med. 1980 Sep 1;152(3):493–506. doi: 10.1084/jem.152.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman G. W., Sultzer B. M. Further studies on the activation of lymphocytes by endotoxin protein. J Immunol. 1979 Apr;122(4):1329–1334. [PubMed] [Google Scholar]
- Goodman G. W., Sultzer B. M. Mild alkaline hydrolysis of lipopolysaccharide endotoxin enhances its mitogencity for murine B cells. Infect Immun. 1977 Jul;17(1):205–214. doi: 10.1128/iai.17.1.205-214.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inman J. K. Thymus-independent antigens: the preparation of covalent, hapten-ficoll conjugates. J Immunol. 1975 Feb;114(2 Pt 1):704–709. [PubMed] [Google Scholar]
- Jacobs D. M., Morrison D. C. Stimulation of a T-independent primary anti-hapten response in vitro by TNP-lipopolysaccharide (TNP-LPS). J Immunol. 1975 Jan;114(1 Pt 2):360–364. [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Ly I. A., Mishell R. I. Separation of mouse spleen cells by passage through columns of sephadex G-10. J Immunol Methods. 1974 Aug;5(3):239–247. doi: 10.1016/0022-1759(74)90108-2. [DOI] [PubMed] [Google Scholar]
- Melchers F., Braun V., Galanos C. The lipoprotein of the outer membrane of Escherichia coli: a B-lymphocyte mitogen. J Exp Med. 1975 Aug 1;142(2):473–482. doi: 10.1084/jem.142.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno T., Kageyama M. Separation and characterization of the outer membrane of Pseudomonas aeruginosa. J Biochem. 1978 Jul;84(1):179–191. doi: 10.1093/oxfordjournals.jbchem.a132106. [DOI] [PubMed] [Google Scholar]
- Mond J. J., Mongini P. K., Sieckmann D., Paul W. E. Role of T lymphocytes in the response to TNP-AECM-Ficoll. J Immunol. 1980 Sep;125(3):1066–1070. [PubMed] [Google Scholar]
- Morgan E. L., Walker S. M., Thoman M. L., Weigle W. O. Regulation of the immune response. I. The potentiation of in vivo and in vitro immune responses by Fc fragments. J Exp Med. 1980 Jul 1;152(1):113–123. doi: 10.1084/jem.152.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K., Mizushima S. Effects of heating in dodecyl sulfate solution on the conformation and electrophoretic mobility of isolated major outer membrane proteins from Escherichia coli K-12. J Biochem. 1976 Dec;80(6):1411–1422. doi: 10.1093/oxfordjournals.jbchem.a131414. [DOI] [PubMed] [Google Scholar]
- Namiki S., Igarashi K., Watanabe T. Chemical and biological properties of the carcinostatic protein fraction of Escherichia coli. Gan. 1978 Apr;69(2):151–158. [PubMed] [Google Scholar]
- Persson U. C., Hammarström L. L., Smith C. I. Macrophages are required for the dextran-sulfate induced activation of B lymphocytes. J Immunol. 1977 Sep;119(3):1138–1144. [PubMed] [Google Scholar]
- Plotz P. H., Talal N., Asofsky R. Assignment of direct and facilitated hemolytic plaques in mice to specific immunoglobulin classes. J Immunol. 1968 Apr;100(4):744–751. [PubMed] [Google Scholar]
- Rittenberg M. B., Pratt K. L. Antitrinitrophenyl (TNP) plaque assay. Primary response of Balb/c mice to soluble and particulate immunogen. Proc Soc Exp Biol Med. 1969 Nov;132(2):575–581. doi: 10.3181/00379727-132-34264. [DOI] [PubMed] [Google Scholar]
- Sjöberg O., Andersson J., Möller G. Lipopolysaccharide can substitute for helper cells in the antibody response in vitro. Eur J Immunol. 1972 Aug;2(4):326–331. doi: 10.1002/eji.1830020406. [DOI] [PubMed] [Google Scholar]
- Specter S., Cimprich R., Friedman H., Chedid L. Stimulation of an enhanced in vitro immune response by a synthetic adjuvant, muramyl dipeptide. J Immunol. 1978 Feb;120(2):487–491. [PubMed] [Google Scholar]
- Sultzer B. M., Goodman G. W. Endotoxin protein: a B-cell mitogen and polyclonal activator of C3H/HeJ lymphocytes. J Exp Med. 1976 Sep 1;144(3):821–827. doi: 10.1084/jem.144.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T. The antitumor action of bacterial extract. I. Suppression of mouse ascites tumors by the treatments with extracts of E. coli. Jpn J Exp Med. 1969 Dec;39(6):631–647. [PubMed] [Google Scholar]
- Watson J., Riblet R. Genetic control of responses to bacterial lipopolysaccharides in mice. I. Evidence for a single gene that influences mitogenic and immunogenic respones to lipopolysaccharides. J Exp Med. 1974 Nov 1;140(5):1147–1161. doi: 10.1084/jem.140.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]



