Abstract
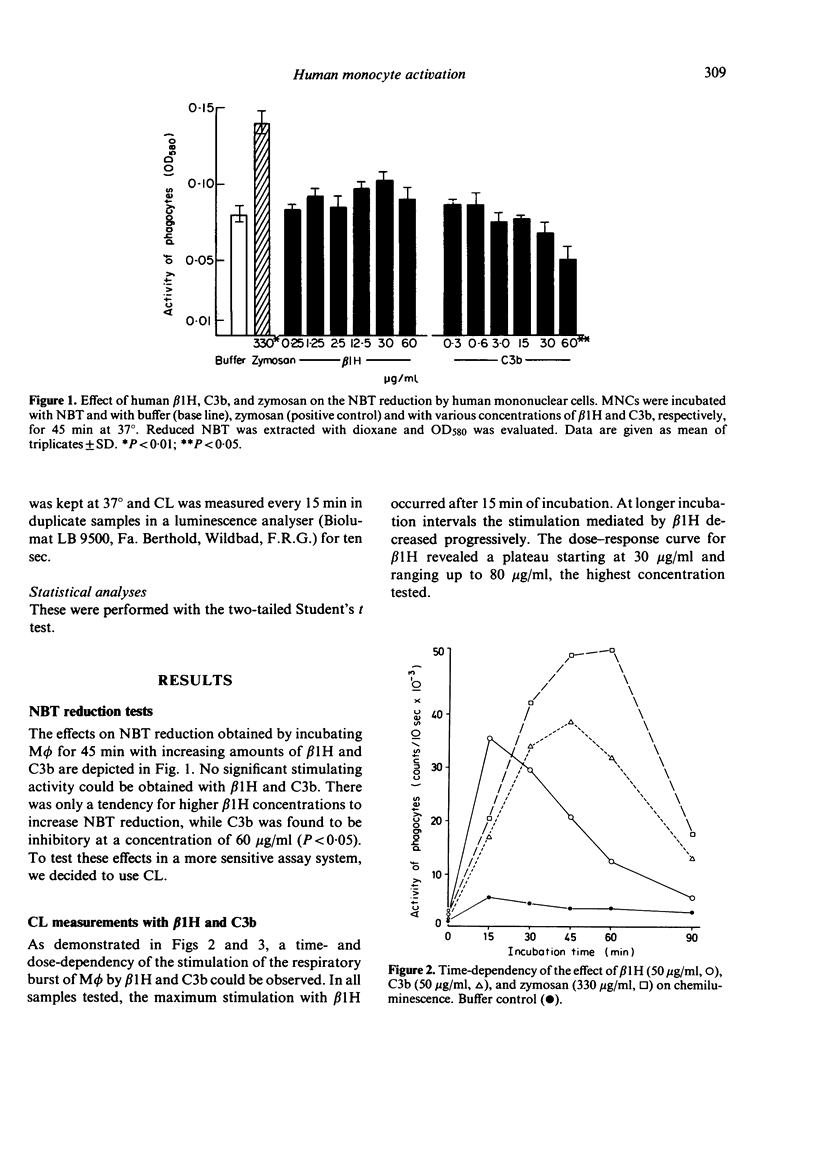
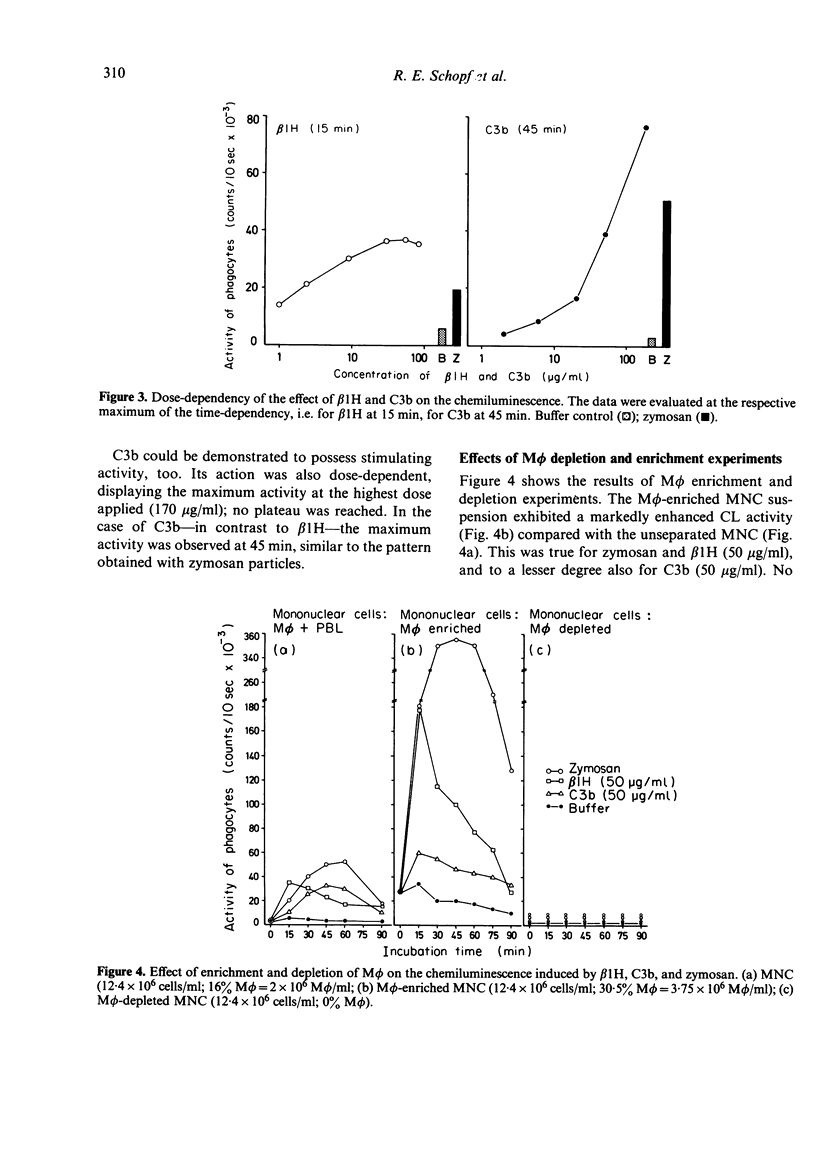
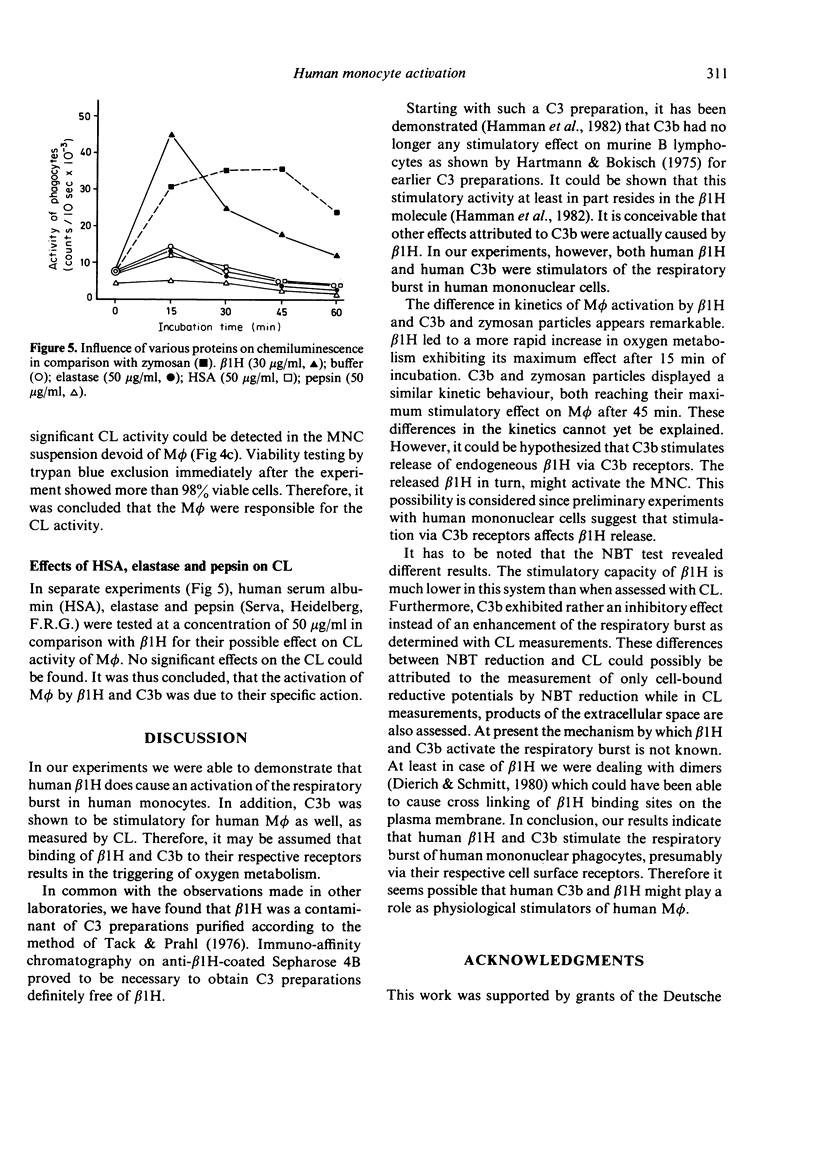
We tested whether highly purified human β1H and C3b, two proteins of the alternative pathway of complement activation, could exert an influence on the activity of human monocytes (Mφ). The activation process of Mφ was assessed by measurements of the respiratory burst in terms of nitro blue tetrazolium (NBT) reduction and by chemiluminescence (CL) tests. In NBT reduction experiments, we found a tendency for β1H to increase NBT reduction, while C3b was found to be rather inhibitory. In CL measurements, both β1H and C3b displayed a stimulatory effect on Mφ, showing different time- and dose-dependency. For β1H, the maximum stimulation occurred after 15 min, whereas for C3b after 45 min. Zymosan particles which served as a positive control also showed the highest stimulation after 45 min. In dose—response experiments, β1H reached a plateau ranging from 30 to 80 μg/ml. In contrast, using C3b up to 170 μg/ml, no plateau was reached. Mφ-depletion and enrichment studies suggested at Mφ as being responsible for the stimulatory effects found.
The differences between NBT reduction and CL could possibly be explained by the measurement of only cell-bound reductive potentials by NBT reduction, while in CL measurements, products of the extracellular space are also assessed. Our results suggest that both human β1H and C3b are appropriate stimuli for human monocytes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. C., Stjernholm R. L., Steele R. H. Evidence for the generation of an electronic excitation state(s) in human polymorphonuclear leukocytes and its participation in bactericidal activity. Biochem Biophys Res Commun. 1972 May 26;47(4):679–684. doi: 10.1016/0006-291x(72)90545-1. [DOI] [PubMed] [Google Scholar]
- Baehner R. L., Nathan D. G. Quantitative nitroblue tetrazolium test in chronic granulomatous disease. N Engl J Med. 1968 May 2;278(18):971–976. doi: 10.1056/NEJM196805022781801. [DOI] [PubMed] [Google Scholar]
- Bokisch V. A., Müller-Eberhard H. J., Cochrane C. G. Isolation of a fragment (C3a) of the third component of human complement containing anaphylatoxin and chemotactic activity and description of an anaphylatoxin inactivator of human serum. J Exp Med. 1969 May 1;129(5):1109–1130. doi: 10.1084/jem.129.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Conrad D. H., Carlo J. R., Ruddy S. Interaction of beta1H globulin with cell-bound C3b: quantitative analysis of binding and influence of alternative pathway components on binding. J Exp Med. 1978 Jun 1;147(6):1792–1805. doi: 10.1084/jem.147.6.1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T. Regulation by membrane sialic acid of beta1H-dependent decay-dissociation of amplification C3 convertase of the alternative complement pathway. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1971–1975. doi: 10.1073/pnas.75.4.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann K. U., Bokisch V. A. Stimulation of murine B lymphocytes by isolated C3b. J Exp Med. 1975 Sep 1;142(3):600–610. doi: 10.1084/jem.142.3.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambris J. D., Dobson N. J., Ross G. D. Release of endogenous C3b inactivator from lymphocytes in response to triggering membrane receptors for beta 1H globulin. J Exp Med. 1980 Dec 1;152(6):1625–1644. doi: 10.1084/jem.152.6.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangburn M. K., Schreiber R. D., Müller-Eberhard H. J. Human complement C3b inactivator: isolation, characterization, and demonstration of an absolute requirement for the serum protein beta1H for cleavage of C3b and C4b in solution. J Exp Med. 1977 Jul 1;146(1):257–270. doi: 10.1084/jem.146.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumpold H., Kraft D., Scheiner O., Radaszkiewicz T. Influence of carbonyl iron treatment on lymphocyte subpopulations. Int Arch Allergy Appl Immunol. 1979;58(1):105–109. doi: 10.1159/000232179. [DOI] [PubMed] [Google Scholar]
- Schmitt M., Mussel H. H., Dierich M. P. Qualitative and quantitative assessment of C3-receptor reactivities on lymphoid and phagocytic cells. J Immunol. 1981 May;126(5):2042–2047. [PubMed] [Google Scholar]
- Schreiber R. D., Pangburn M. K., Lesavre P. H., Müller-Eberhard H. J. Initiation of the alternative pathway of complement: recognition of activators by bound C3b and assembly of the entire pathway from six isolated proteins. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3948–3952. doi: 10.1073/pnas.75.8.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tack B. D., Prahl J. W. Third component of human complement: purification from plasma and physicochemical characterization. Biochemistry. 1976 Oct 5;15(20):4513–4521. doi: 10.1021/bi00665a028. [DOI] [PubMed] [Google Scholar]
- Tucker S. B., Pierre R. V., Jordon R. E. Rapid identification of monocytes in a mixed mononuclear cell preparation. J Immunol Methods. 1977;14(3-4):267–269. doi: 10.1016/0022-1759(77)90137-5. [DOI] [PubMed] [Google Scholar]
- Weissmann G., Smolen J. E., Korchak H. M. Release of inflammatory mediators from stimulated neutrophils. N Engl J Med. 1980 Jul 3;303(1):27–34. doi: 10.1056/NEJM198007033030109. [DOI] [PubMed] [Google Scholar]
- Zembala M., Lemmel E. M., Uracz W. Activation of human monocytes for nitroblue tetrazolium reduction and the suppression of lymphocyte response to mitogens. Clin Exp Immunol. 1980 Aug;41(2):309–316. [PMC free article] [PubMed] [Google Scholar]


