Abstract
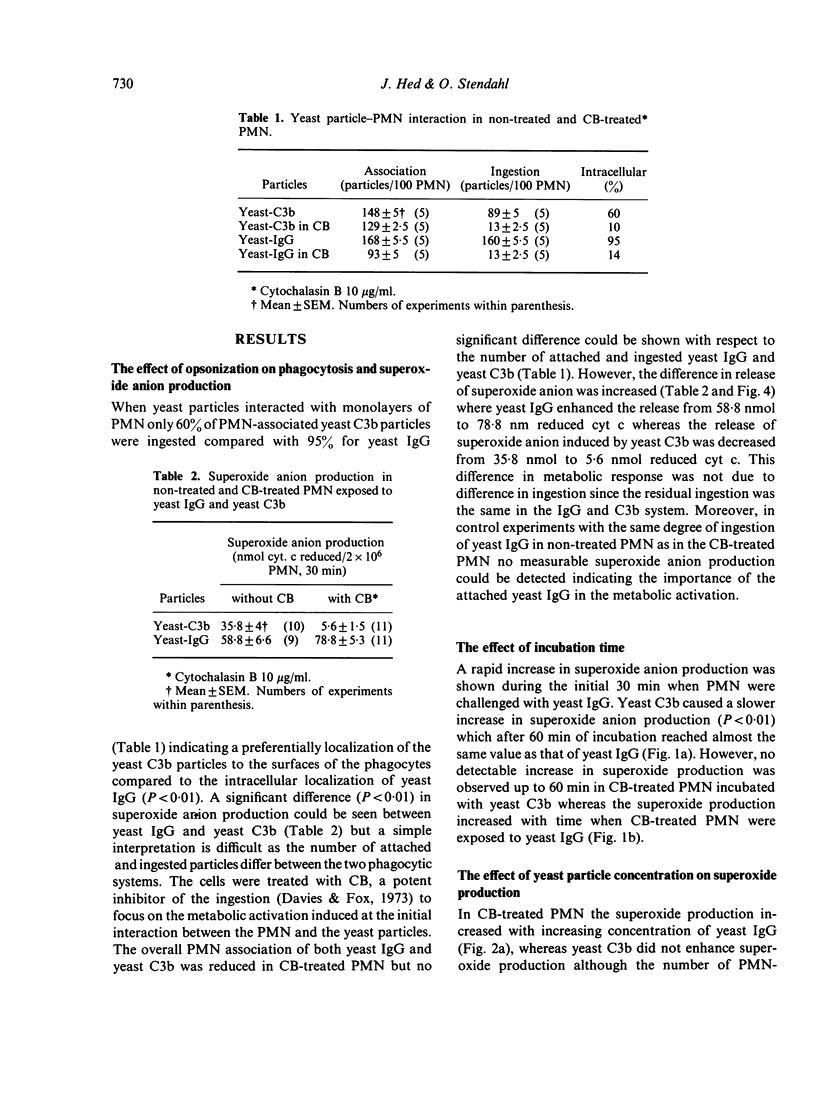
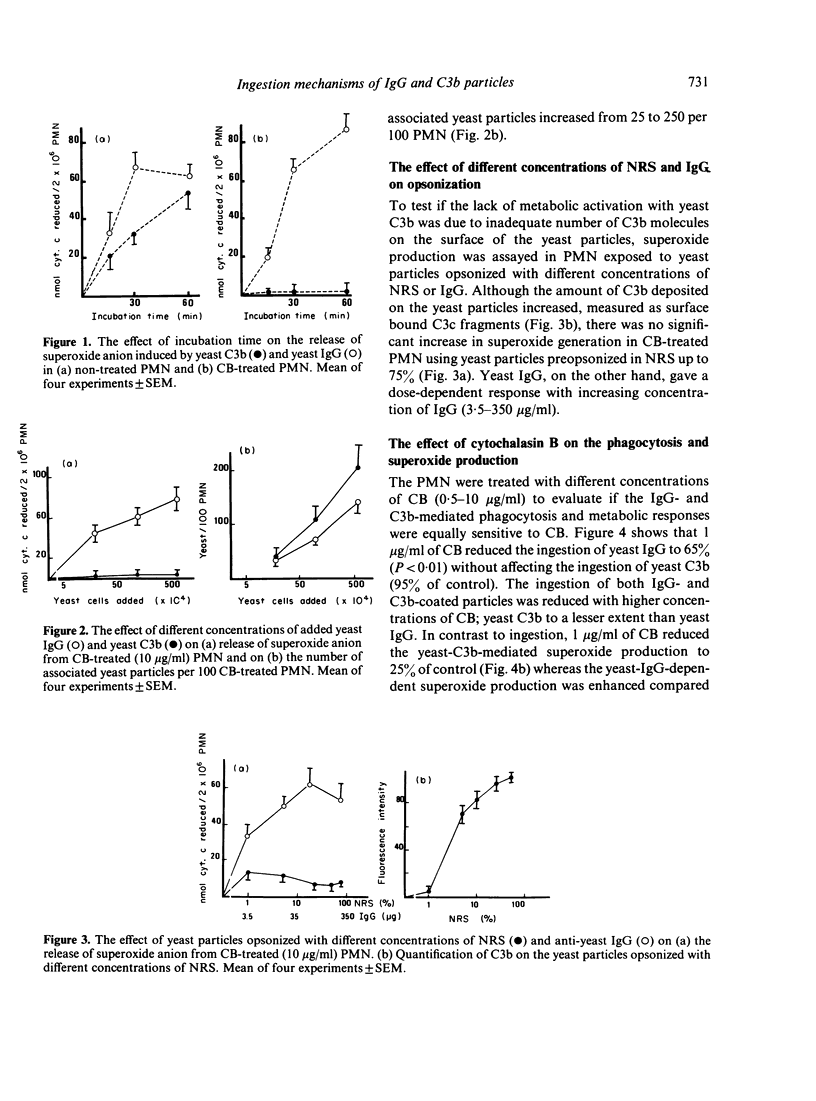
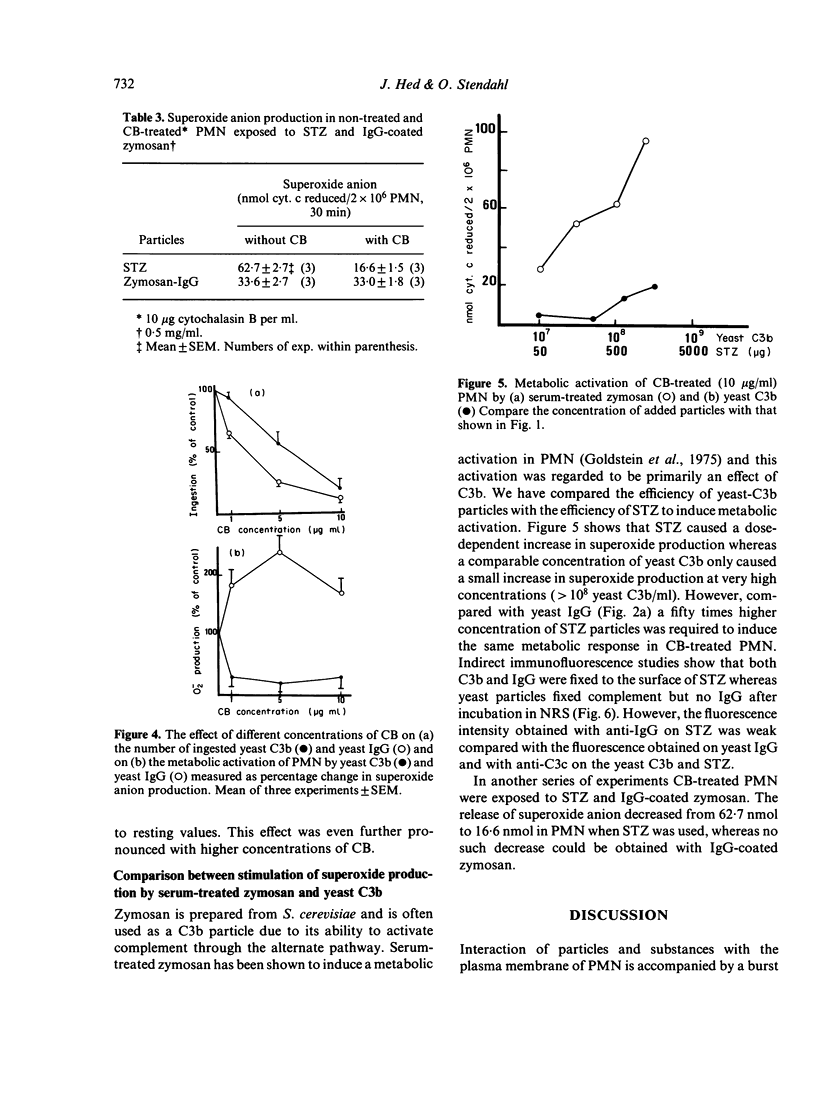
A prominent feature of the surface interaction between the phagocyte and particles coated with IgG and C3b is its remarkably discriminatory character. IgG primarily promotes ingestion whereas C3b primarily promotes attachment. However, in several systems the C3b molecule is reported to cause membrane pertubation as efficiently as the IgG molecule. In this study we have used yeast particles coated with specific IgG or C3b as preys in a newly developed phagocytic assay. The number of particles interacting with polymorphonuclear leucocytes (PMN) was correlated with effector responses monitored as percentage ingested particles, superoxide anion production, and sensitivity of the ingestion process to cytochalasin B. During phagocytosis only 60% of PMN-associated yeast-C3b particles were ingested compared with 95% of yeast IgG. In cytochalasin B treated PMN, where ingestion was virtually abolished, attached yeast IgG-induced superoxide anion production whereas the same number of attached yeast C3b did not. Attempts to induce superoxide anion production in the C3b system failed by increasing incubation time, number of added particles or by increasing the concentration of the opsonizing protein. A low concentration of cytochalasin B (1 μg/ml) decreased the IgG-dependent ingestion to 65% without affecting the C3b-dependent ingestion. The results indicate the existence of different ingestion processes in PMN—one more `basal' ingestion process represented by the C3b-promoted ingestion independent of membrane activation and another `active' ingestion process represented by the IgG-promoted ingestion dependent on membrane activation and an active response of the PMN.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C., Davies P. Mechanisms of endocytosis and exocytosis. Symp Soc Exp Biol. 1974;(28):419–446. [PubMed] [Google Scholar]
- Bianco C., Griffin F. M., Jr, Silverstein S. C. Studies of the macrophage complement receptor. Alteration of receptor function upon macrophage activation. J Exp Med. 1975 Jun 1;141(6):1278–1290. doi: 10.1084/jem.141.6.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxer L. A., Hedley-Whyte E. T., Stossel T. P. Neutrophil action dysfunction and abnormal neutrophil behavior. N Engl J Med. 1974 Nov 21;291(21):1093–1099. doi: 10.1056/NEJM197411212912101. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Curnutte J. T., Babior B. M. Biological defense mechanisms. The effect of bacteria and serum on superoxide production by granulocytes. J Clin Invest. 1974 Jun;53(6):1662–1672. doi: 10.1172/JCI107717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies P., Fox R. I., Polyzonis M., Allison A. C., Haswell A. D. The inhibition of phagocytosis and facilitation of exocytosis in rabbit polymorphonuclear leukocytes by cytochalasin B. Lab Invest. 1973 Jan;28(1):16–22. [PubMed] [Google Scholar]
- Ehlenberger A. G., Nussenzweig V. The role of membrane receptors for C3b and C3d in phagocytosis. J Exp Med. 1977 Feb 1;145(2):357–371. doi: 10.1084/jem.145.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine D. P., Marney S. R., Jr, Colley D. G., Sergent J. S., Des Prez R. M. C3 shunt activation in human serum chelated with EGTA. J Immunol. 1972 Oct;109(4):807–809. [PubMed] [Google Scholar]
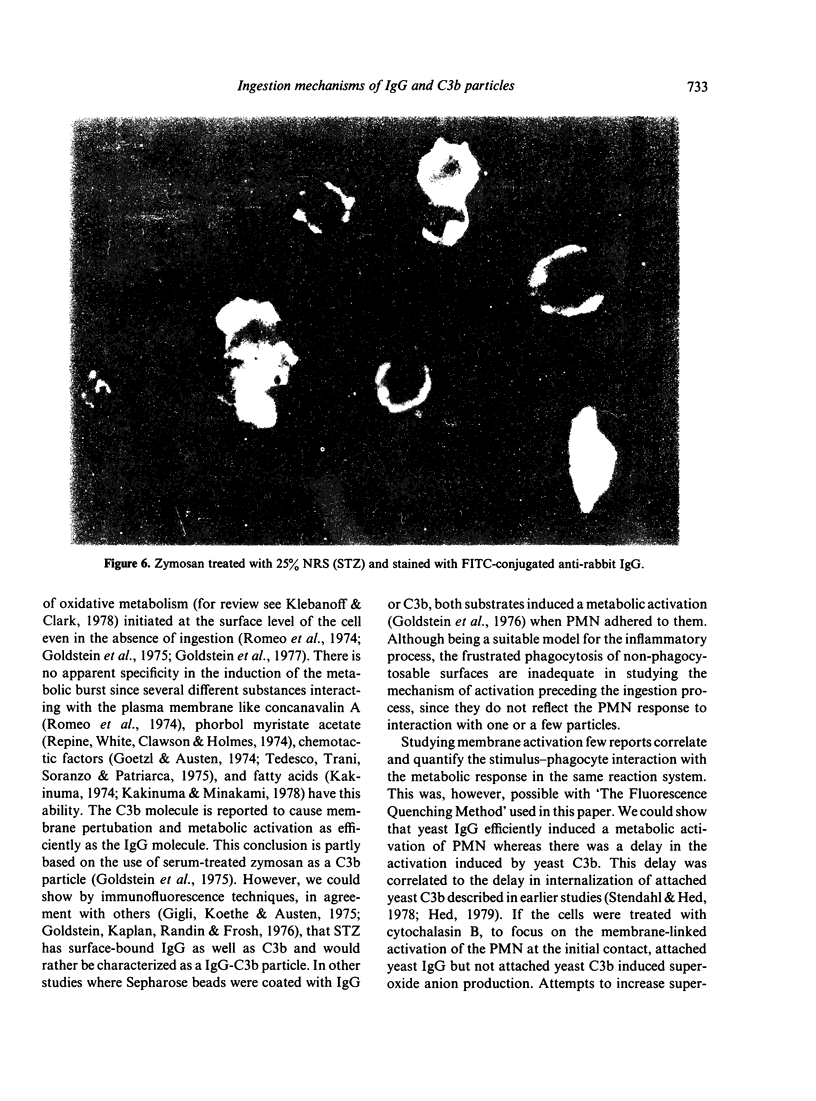
- Gigli I., Koethe S., Austen K. F. Participation of an early component of complement and Hageman factor in C3 destruction by zymosan. Clin Immunol Immunopathol. 1975 Jul;4(2):189–198. doi: 10.1016/0090-1229(75)90054-9. [DOI] [PubMed] [Google Scholar]
- Goetzl E. J., Austen K. F. Stimulation of human neutrophil leukocyte aerobic glucose metabolism by purified chemotactic factors. J Clin Invest. 1974 Feb;53(2):591–599. doi: 10.1172/JCI107594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein I. M., Cerqueira M., Lind S., Kaplan H. B. Evidence that the superoxide-generating system of human leukocytes is associated with the cell surface. J Clin Invest. 1977 Feb;59(2):249–254. doi: 10.1172/JCI108635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein I. M., Kaplan H. B., Radin A., Frosch M. Independent effects of IgG and complement upon human polymorphonuclear leukocyte function. J Immunol. 1976 Oct;117(4):1282–1287. [PubMed] [Google Scholar]
- Griffin F. M., Jr, Griffin J. A., Leider J. E., Silverstein S. C. Studies on the mechanism of phagocytosis. I. Requirements for circumferential attachment of particle-bound ligands to specific receptors on the macrophage plasma membrane. J Exp Med. 1975 Nov 1;142(5):1263–1282. doi: 10.1084/jem.142.5.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwig J. H., Stossel T. P. Cytochalasin B and the structure of actin gels. J Mol Biol. 1979 Nov 5;134(3):539–553. doi: 10.1016/0022-2836(79)90366-8. [DOI] [PubMed] [Google Scholar]
- Huber H., Polley M. J., Linscott W. D., Fudenberg H. H., Müller-Eberhard H. J. Human monocytes: distinct receptor sites for the third component of complement and for immunoglobulin G. Science. 1968 Dec 13;162(3859):1281–1283. doi: 10.1126/science.162.3859.1281. [DOI] [PubMed] [Google Scholar]
- Kaplan G. Differences in the mode of phagocytosis with Fc and C3 receptors in macrophages. Scand J Immunol. 1977;6(8):797–807. doi: 10.1111/j.1365-3083.1977.tb02153.x. [DOI] [PubMed] [Google Scholar]
- Klaus G. G. Cytochalasin B. Dissociation of pinocytosis and phagocytosis by peritoneal macrophages. Exp Cell Res. 1973 Apr;79(1):73–78. doi: 10.1016/0014-4827(73)90490-4. [DOI] [PubMed] [Google Scholar]
- Kärre K., Seeley J. K. Cytotoxic Thy 1,2-positive blasts with NK-like target selectivity in murine mixed lymphocyte cultures. J Immunol. 1979 Oct;123(4):1511–1518. [PubMed] [Google Scholar]
- Lay W. H., Nussenzweig V. Receptors for complement of leukocytes. J Exp Med. 1968 Nov 1;128(5):991–1009. doi: 10.1084/jem.128.5.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARGOLIASH E., FROHWIRT N. Spectrum of horse-heart cytochrome c. Biochem J. 1959 Mar;71(3):570–572. doi: 10.1042/bj0710570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson K. E., Dahlgren C., Stendahl O., Sundqvist T. Characteristics of the phagocytic process assessed by Coulter Counter. Acta Pathol Microbiol Scand C. 1977 Jun;85(3):215–221. doi: 10.1111/j.1699-0463.1977.tb03633.x. [DOI] [PubMed] [Google Scholar]
- Mantovani B. Different roles of IgG and complement receptors in phagocytosis by polymorphonuclear leukocytes. J Immunol. 1975 Jul;115(1):15–17. [PubMed] [Google Scholar]
- Mantovani B., Rabinovitch M., Nussenzweig V. Phagocytosis of immune complexes by macrophages. Different roles of the macrophage receptor sites for complement (C3) and for immunoglobulin (IgG). J Exp Med. 1972 Apr 1;135(4):780–792. doi: 10.1084/jem.135.4.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munthe-Kaas A. C., Kaplan G., Seljelid R. On the mechanism of internalization of opsonized particles by rat Kupffer cells in vitro. Exp Cell Res. 1976 Nov;103(1):201–212. doi: 10.1016/0014-4827(76)90256-1. [DOI] [PubMed] [Google Scholar]
- Rabellino E. M., Ross G. D., Polley M. J. Membrane receptors of mouse leukocytes. I. Two types of complement receptors for different regions of C3. J Immunol. 1978 Mar;120(3):879–885. [PubMed] [Google Scholar]
- Repine J. E., White J. G., Clawson C. C., Holmes B. M. Effects of phorbol myristate acetate on the metabolism and ultrastructure of neutrophils in chronic granulomatous disease. J Clin Invest. 1974 Jul;54(1):83–90. doi: 10.1172/JCI107752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo D., Jug M., Zabucchi G., Rossi F. Perturbation of leukocyte metabolism by nonphagocytosable concanavalin A-coupled beads. FEBS Lett. 1974 May 15;42(1):90–93. doi: 10.1016/0014-5793(74)80286-3. [DOI] [PubMed] [Google Scholar]
- Scribner D. J., Fahrney D. Neutrophil receptors for IgG and complement: their roles in the attachment and ingestion phases of phagocytosis. J Immunol. 1976 Apr;116(4):892–897. [PubMed] [Google Scholar]
- Stendahl O., Hed J., Kihlström E., Magnusson K. E., Tagesson C. Phagocytic internalization and the requirement for membrane perturbation. FEBS Lett. 1977 Sep 1;81(1):118–120. doi: 10.1016/0014-5793(77)80941-1. [DOI] [PubMed] [Google Scholar]
- Stossel T. P., Field R. J., Gitlin J. D., Alper C. A., Rosen F. S. The opsonic fragment of the third component of human complement (C3). J Exp Med. 1975 Jun 1;141(6):1329–1347. doi: 10.1084/jem.141.6.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedesco F., Trani S., Soranzo M. R., Patriarca P. Stimulation of glucose oxidation in human polymorphonuclear leucocytes by C3-Sepharose and soluble C567. FEBS Lett. 1975 Mar 1;51(1):232–236. doi: 10.1016/0014-5793(75)80894-5. [DOI] [PubMed] [Google Scholar]
- Tsan M. F., Douglass K. H., McIntyre P. A. Hydrogen peroxide production and killing of Staphylococcus aureus by human polymorphonuclear leukocytes. Blood. 1977 Mar;49(3):437–444. [PubMed] [Google Scholar]
- Weening R. S., Roos D., Weemaes C. M., Homan-Müller J. W., van Schaik M. L. Defective initiation of the metabolic stimulation in phagocytizing granulocytes: a new congenital defect. J Lab Clin Med. 1976 Nov;88(5):757–768. [PubMed] [Google Scholar]
- Yagawa K., Onoue K., Aida Y. Structural studies of Fc receptors. I. Binding properties, solubilization, and partial characterization of fc receptors of macrophages. J Immunol. 1979 Jan;122(1):366–373. [PubMed] [Google Scholar]
- Yamamura M., Valdimarsson H. A new semiquantitative radiometric opsonin assay. Selective measurement of opsonizing capacity of the alternative pathway. Immunology. 1978 Apr;34(4):689–694. [PMC free article] [PubMed] [Google Scholar]