Abstract
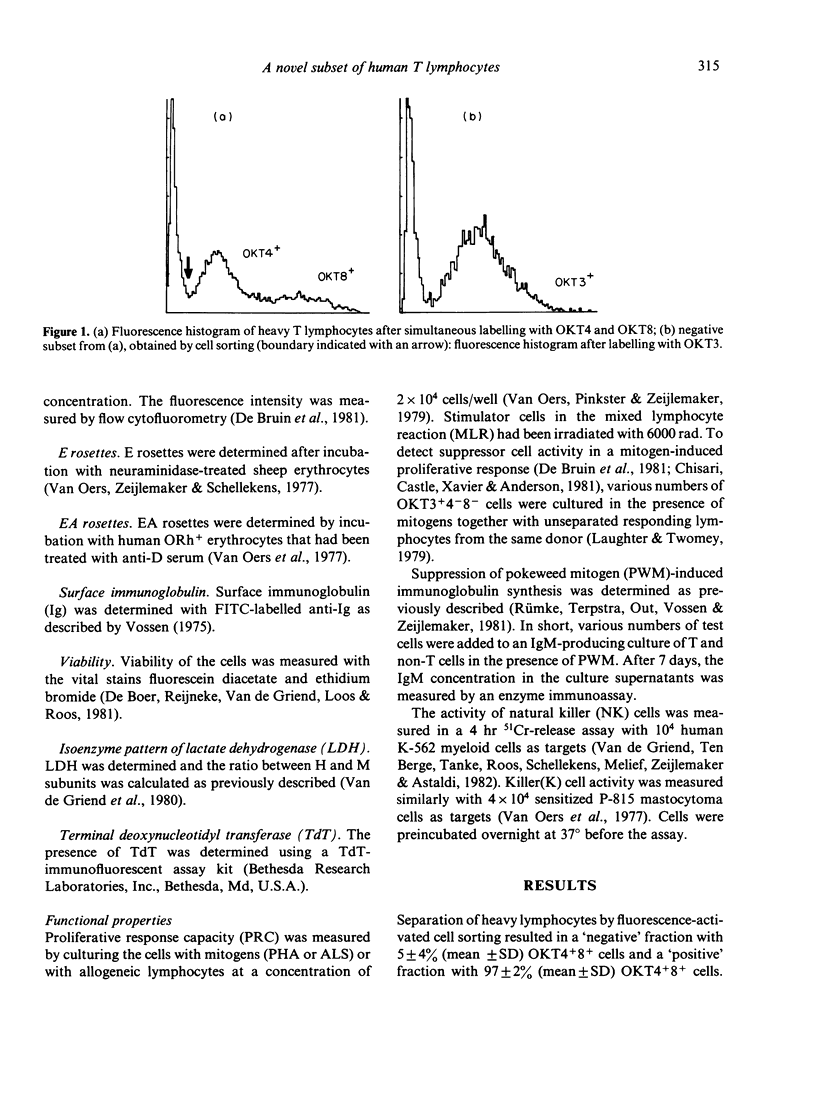
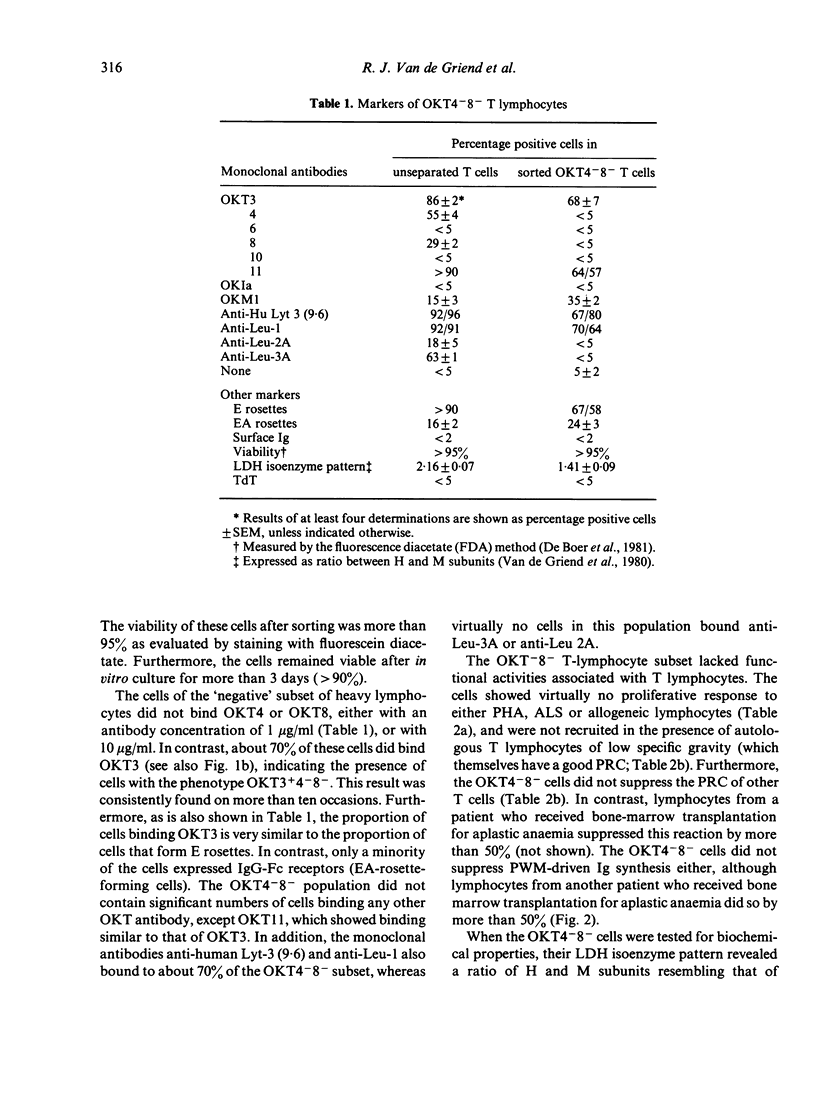
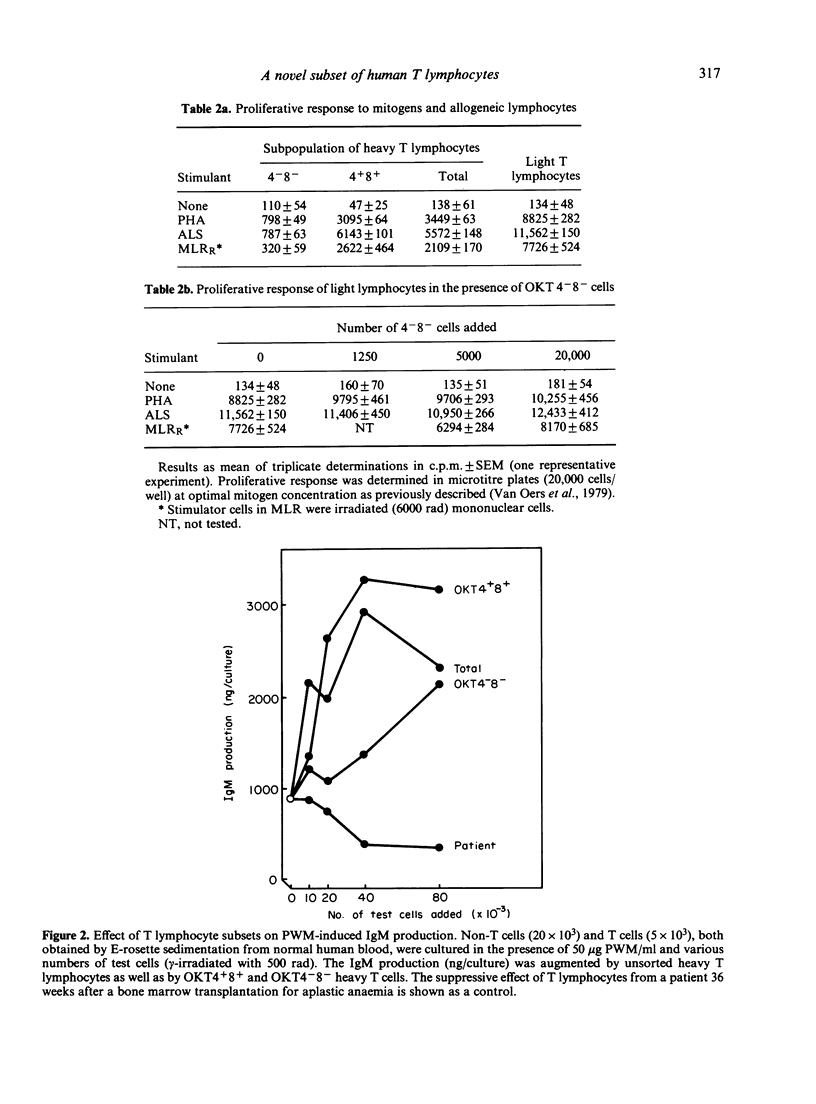
A novel subset of human blood lymphocytes was isolated by means of labelling with monoclonal antibodies and fluorescence-activated cell sorting. In normal individuals, the new subset accounts for about 2% of the blood T lymphocytes. The cells of this subset bind monoclonal antibodies specific for T lymphocytes in general [e.g. OKT3, Hu-Lyt 3(9 . 6) and Leu-22] and they also form E rosettes. However, no binding is seen with monoclonal antibodies to T-lymphocyte subsets (OKT4, OKT8, Leu-2A and Leu-3A). Moreover, the lymphocytes of this new subset express neither Ia antigens nor membrane immunoglobulins. They do not bind OKM1, an antibody against cells of the myelomonocytic lineage that also reacts with natural killer cells, nor do they bind OKT6 or OKT10, specific for thymocyte antigens. The cells have a high specific gravity, a thymocyte-like pattern of lactate dehydrogenase isoenzymes and do not contain terminal deoxynucleotidyl transferase. Although these lymphocytes are viable, also after culture in vitro, and can be stored in liquid nitrogen, they are inert in all functional systems tested: they neither proliferate upon stimulation with mitogens or allogeneic cells, nor do they display suppressor or natural killer cell activity. A patient who was successfully reconstituted by bone marrow transplantation for severe combined immunodeficiency, was found to contain an abnormally high (25%--30%) fraction of these OKT3 positive, OKT4 and OKT8 negative cells among his circulating T lymphocytes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Breard J., Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. A monoclonal antibody reactive with human peripheral blood monocytes. J Immunol. 1980 Apr;124(4):1943–1948. [PubMed] [Google Scholar]
- Breard J., Reinherz E. L., O'Brien C., Schlossman S. F. Delineation of an effector population responsible for natural killing and antibody-dependent cellular cytotoxicity in man. Clin Immunol Immunopathol. 1981 Jan;18(1):145–150. doi: 10.1016/0090-1229(81)90018-0. [DOI] [PubMed] [Google Scholar]
- Chisari F. V., Castle K. L., Xavier C., Anderson D. S. Functional properties of lymphocyte subpopulations in hepatitis B virus infection. I. Suppressor cell control of T lymphocyte responsiveness. J Immunol. 1981 Jan;126(1):38–44. [PubMed] [Google Scholar]
- De Boer M., Reijneke R., Van de Griend R. J., Loos J. A., Roos D. Large-scale purification and cryopreservation of human monocytes. J Immunol Methods. 1981;43(2):225–239. doi: 10.1016/0022-1759(81)90027-2. [DOI] [PubMed] [Google Scholar]
- Fox R. I., Thompson L. F., Huddlestone J. R. T gamma cells express T lymphocyte-associated antigens. J Immunol. 1981 May;126(5):2062–2063. [PubMed] [Google Scholar]
- Hoffman R. A., Kung P. C., Hansen W. P., Goldstein G. Simple and rapid measurement of human T lymphocytes and their subclasses in peripheral blood. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4914–4917. doi: 10.1073/pnas.77.8.4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamoun M., Martin P. J., Hansen J. A., Brown M. A., Siadak A. W., Nowinski R. C. Identification of a human T lymphocyte surface protein associated with the E-rosette receptor. J Exp Med. 1981 Jan 1;153(1):207–212. doi: 10.1084/jem.153.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay H. D., Horwitz D. A. Evidence by reactivity with hybridoma antibodies for a probable myeloid origin of peripheral blood cells active in natural cytotoxicity and antibody-dependent cell-mediated cytotoxicity. J Clin Invest. 1980 Oct;66(4):847–851. doi: 10.1172/JCI109923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung P. C., Talle M. A., DeMaria M. E., Butler M. S., Lifter J., Goldstein G. Strategies for generating monoclonal antibodies defining human t-lymphocyte differentiation antigens. Transplant Proc. 1980 Sep;12(3 Suppl 1):141–146. [PubMed] [Google Scholar]
- Kung P., Goldstein G., Reinherz E. L., Schlossman S. F. Monoclonal antibodies defining distinctive human T cell surface antigens. Science. 1979 Oct 19;206(4416):347–349. doi: 10.1126/science.314668. [DOI] [PubMed] [Google Scholar]
- Laughter A. H., Twomey J. J. Suppression of lymphoproliferation by high concentrations of normal human mononuclear leukocytes. J Immunol. 1977 Jul;119(1):173–179. [PubMed] [Google Scholar]
- Ledbetter J. A., Evans R. L., Lipinski M., Cunningham-Rundles C., Good R. A., Herzenberg L. A. Evolutionary conservation of surface molecules that distinguish T lymphocyte helper/inducer and cytotoxic/suppressor subpopulations in mouse and man. J Exp Med. 1981 Feb 1;153(2):310–323. doi: 10.1084/jem.153.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loos J. A., Roos D. Ficoll-isopaque gradients for the determination of density distributions of human blood lymphocytes and other reticulo-endothelial cells. Exp Cell Res. 1974 Jun;86(2):333–341. doi: 10.1016/0014-4827(74)90721-6. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Levey R. H., Schlossman S. F. Discrete stages of human intrathymic differentiation: analysis of normal thymocytes and leukemic lymphoblasts of T-cell lineage. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1588–1592. doi: 10.1073/pnas.77.3.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. A monoclonal antibody reactive with the human cytotoxic/suppressor T cell subset previously defined by a heteroantiserum termed TH2. J Immunol. 1980 Mar;124(3):1301–1307. [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. Separation of functional subsets of human T cells by a monoclonal antibody. Proc Natl Acad Sci U S A. 1979 Aug;76(8):4061–4065. doi: 10.1073/pnas.76.8.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Moretta L., Roper M., Breard J. M., Mingari M. C., Cooper M. D., Schlossman S. F. Human T lymphocyte subpopulations defined by Fc receptors and monoclonal antibodies. A comparison. J Exp Med. 1980 Apr 1;151(4):969–974. doi: 10.1084/jem.151.4.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Schlossman S. F. The differentiation and function of human T lymphocytes. Cell. 1980 Apr;19(4):821–827. doi: 10.1016/0092-8674(80)90072-0. [DOI] [PubMed] [Google Scholar]
- Roos D., Loos J. A. Changes in the carbohydrate metabolism of mitogenically stimulated human peripheral lymphocytes. I. Stimulation by phytohaemagglutinin. Biochim Biophys Acta. 1970 Dec 29;222(3):565–582. doi: 10.1016/0304-4165(70)90182-0. [DOI] [PubMed] [Google Scholar]
- Rümke H. C., Terpstra F. G., Out T. A., Vossen J. M., Zeijlemaker W. P. Immunoglobulin production by human lymphocytes in a microculture system: culture conditions and cellular interactions. Clin Immunol Immunopathol. 1981 Jun;19(3):338–350. doi: 10.1016/0090-1229(81)90077-5. [DOI] [PubMed] [Google Scholar]
- Zarling J. M., Kung P. C. Monoclonal antibodies which distinguish between human NK cells and cytotoxic T lymphocytes. Nature. 1980 Nov 27;288(5789):394–396. doi: 10.1038/288394a0. [DOI] [PubMed] [Google Scholar]
- de Bruin H. G., Astaldi A., Leupers T., van de Griend R. J., Dooren L. J., Schellekens P. T., Tanke H. J., Roos M., Vossen J. M. T lymphocyte characteristics in bone marrow-transplanted patients. II. Analysis with monoclonal antibodies. J Immunol. 1981 Jul;127(1):244–251. [PubMed] [Google Scholar]
- van Oers M. H., Zeijlemaker W. P., Schellekens P. T. Separation and properties of EA-rosette-forming lymphocytes in humans. Eur J Immunol. 1977 Mar;7(3):143–150. doi: 10.1002/eji.1830070306. [DOI] [PubMed] [Google Scholar]
- van de Griend R. J., Astaldi G. C., van den Ende A., Loos J. A., Roos D. Subpopulations of T lymphocytes from human blood differing in density and stage of maturation. Eur J Immunol. 1980 Jan;10(1):70–73. doi: 10.1002/eji.1830100114. [DOI] [PubMed] [Google Scholar]
- van de Griend R. J., ten Berge I., Tanke H. J., Roos D., Schellekens P. T., Melief C. J., Zeijlemaker W. P., Astaldi A. Characterization of two subsets of human T gamma cells. J Immunol. 1982 May;128(5):1979–1985. [PubMed] [Google Scholar]
- van de Griend R., Astaldi A., de Bruin H., Tanke H., van Doorn R., Meerhof L., Roos D. Characterization of physically different human T-cell subsets with monoclonal antibodies and biochemical markers. Clin Immunol Immunopathol. 1981 Oct;21(1):94–105. doi: 10.1016/0090-1229(81)90198-7. [DOI] [PubMed] [Google Scholar]


