Abstract
Background
The human malaria parasite Plasmodium falciparum expresses adhesins belonging to the erythrocyte membrane protein 1 (PfEMP1) family on the surface of the infected host erythrocyte. These antigens elicit a strain-specific antibody response that is associated with protection from disease. During clonal expansion of blood-stage parasites, the surface phenotype of the infected erythrocyte changes because of transcriptional switching among the 40 to 50 members of the highly polymorphic var multi-gene family which encode PfEMP1 variants. Studies to date have compared var repertoires of natural isolates from various geographical locations but have not addressed any within-population structure that may exist among repertoires.
Methods
Distinct parasite genotypes from a single population co-circulating among a defined group of hosts were selected. PCR products encoding the DBL-α domain of PfEMP-1 were cloned and sequenced from each of three isolates. Repertoire similarity was statistically evaluated using combinatorial analysis. The chromosomal location of shared sequences was inferred from similarity to dbl-α of known location in the 3D7 genome.
Results
Sympatric parasites were found to share few var gene sequences, even when alleles at other polymorphic loci were shared. A number of the sequences shared by at least two of the isolates studied were found to be related to 3D7 genomic sequences with non-telomeric chromosomal locations, or atypical domain structures, which may represent globally conserved loci.
Conclusion
The parasite population studied is structured, with minimal overlap in PfEMP1 repertoires. The var gene family accumulates diversity more rapidly than other antigen genes examined. This may be facilitated by ectopic recombination among the sub-telomeric regions of P. falciparum chromosomes.
Background
Human erythrocytes infected with certain life-cycle stages of the malaria parasite Plasmodium falciparum express on their surface a family of parasite-encoded adhesins known collectively as PfEMP1, encoded by 40 to 50 members of the var multi-gene family [1]. The extra-cellular portion of PfEMP1 proteins is made up of multiple domains [1] which mediate cytoadherence to a wide range of receptors in host tissues, permitting sequestration out of the peripheral circulation. One class of adhesive domain is named "Duffy-binding like" (DBL) because of similarity to the ligand that binds the Duffy antigen on the surface of the host reticulocyte/erythrocyte during invasion in certain species of Plasmodium (see references in [2]). Each var gene potentially encodes between one and six DBL domains. The amino-terminal DBL-α is the most common extra-cellular domain, occurring in all but a handful of PfEMP1 polypeptide sequences predicted to date [2,3]. var sequence similarity does not necessarily indicate shared geographic origin, and substantial dbl-α sequence polymorphism is found at the genomic level among parasite isolates both within and among endemic areas [6,7].
PfEMP1 elicits an antibody response which develops rapidly in the course of an infection but is of finite duration [8,9]. These strain-specific agglutinating antibodies are the only humoral immune responses to P. falciparum strongly associated with protection from malaria disease in humans [10,11]. The parasite evades the host immune response against PfEMP1 by clonal switching of transcription among var genes encoding the variants on the erythrocyte surface. This process of antigenic variation extends the duration of infection with any particular P. falciparum genotype, as a small proportion of each new generation of progeny expresses novel surface phenotypes not recognised by concurrent anti-PfEMP1 antibodies.
It has been suggested that such protective, variant-specific host immune responses may impose a strain structure upon P. falciparum, as observed in some populations of prokaryotic pathogens such as Neisseria meningitidis [11]. Such strain complexes would be characterised by little or no overlap in expressed PfEMP1 repertoire or Varotype [2]. Studies of var gene repertoires to date have not adequately tested this hypothesis for several reasons. Firstly, most have examined diverse collections of clinical isolates [4-7] rather than parasite genotypes co-circulating among the same hosts. Secondly, the multi-clonal infections characteristic of most malaria-endemic areas frequently prevent resolution of individual var gene repertoires from single isolates [2,6]. Thirdly, as circulating antibodies specific for the infected erythrocyte surface may not persist for long after a particular infection is resolved [8], any immunity-induced strain structure is likely to be transient. Thus any attempt to test the effect of immune selection on P. falciparum strain structure by the definition of var repertoires [2,11] requires examination of monoclonal parasite isolates circulating among a defined human host population at one particular time.
We have been investigating genetic diversity in the P. falciparum population circulating among the Yekwana and Yanomami peoples of the Padamo Basin, Amazon region, Venezuela [12]. Malaria is meso-endemic, with a level of transmission comparable to that in Sudan. Genetic diversity among the parasite population is low, there is significant linkage disequilibrium among antigen loci, and a dominant clone accounted for the bulk of transmission in each of two seasons examined [12]. We selected three monoclonal isolates previously characterised at a number of polymorphic loci, and determined the sequence of between 14 and 32 dbl-α sequences from each. The three dbl-α repertoires were then examined for shared identical sequences. We demonstrate that these three isolates have minimal overlap and discuss these findings in terms of the contribution of both ectopic recombination and immune selection to the generation of PfEMP1 diversity in P. falciparum.
Methods
Study subjects
In a cross-sectional survey of all members of nine communities in the Padamo River basin, Amazon region, Venezuela between November 1995 and February 1996 (n = 708), 43 (6%) individuals were slide-positive for P. falciparum. The genotype of 41 of these was determined at the single-locus genes (msp-1, msp-2, glurp) and the multi-locus gene family (rif) [12]. 40 of the isolates were deemed monoclonal in that they carried only one allele of each of the single-copy loci tested.
Samples and nucleic acid extraction
Isolates were collected and stored as described [12]. DNA extractions were performed as previously described [12].
Amplification and purification of PCR products, cloning in plasmids and sequencing
We amplified dbl-α sequences from purified isolate DNA using the α-AF and α-BR primers of Taylor et al.[4]. The DNA bands (approximately 400 ± 50 bp) representing amplified dbl-α sequences were excised from agarose gels, purified, cloned into plasmids and sequenced as described previously [12]. Sequence data were viewed and annotated using Chromas software http://www.technelysium.com.au. Sixty-one sequences described have been deposited in the EMBL Nucleotide Sequence Database and have the accession numbers AJ536694 to AJ536754.
Data analysis
Sequence data were edited, translated and compared using a variety of bioinformatic tools available at the Baylor College of Medicine http://www.hgsc.bcm.tmc.edu/SearchLauncher and the ExPAsy site http://www.expasy.ch/tools/dna.html. Predicted dbl-α amino acid sequences were compared with those derived from the annotated genomic sequence of the laboratory clone 3D7 using BLAST software at the PlasmoDB site, Release 4.0 http://www.plasmodb.org.
Repertoire overlap was investigated in a pair-wise fashion using combinatorial probabilities. Assuming 58 dbl-α copies per genome as in laboratory clone 3D7 [3], we tested the null hypothesis that the two isolates being compared have dbl-α repertoires drawn randomly from a single pool, size N = 58. If a sequences were obtained from isolate A, we have the proportion p = a/58 of the full dbl-α repertoire of isolate A. Then if we have b sequences from isolate B, of which r are identical to sequences in isolate A, the probability of this outcome (ie r successes from b trials) if the null hypothesis is true, is derived directly from the binomial distribution by:
P (r) = b!/{r!(b - r)!} × pr(1 - p) [b - r] (as described in [13]).
Results
Similarity between var gene repertoires
Three monoclonal isolates that had been collected within six weeks of each other were selected. Isolate genotypes at three polymorphic loci and the rif / rrm fingerprint type of each are shown in Table 1, together with their relative frequencies. These 3 multi-locus genotypes together accounted for 80% of the 40 isolates collected in 1996, The reference isolate (#1; from a male of 13 years) represents the most common genotype [12]. Isolate #2 (from a male of 40 years living 20 km away) is identical at the single-copy polymorphic loci msp-1, msp-2 and glurp to isolate #1, but differs in rif fingerprint [12]. Isolate #3 (from a female of 36 years living near the host of isolate #1) differs from both #1 and #2 at msp-1, msp-2, glurp and rif.
Table 1.
Genotype of three P. falciparum isolates described in this study, and by Tami et al. (2002).
| Isolate | GLURP | MSP1* | MSP2 | RRM type | Relative frequency among 40 isolates** |
| #1 | 1000 bp | K1 232 bp | IC 535 bp | Ia | 62.5% |
| #2 | 1000 bp | K1 232 bp | IC 535 bp | Ib | 12.5% |
| #3 | 883 bp | MAD20 155 bp | IC 523 bp | II | 5% |
*K1 and MAD20 represent distinct allelic families of MSP1 sequences; IC represents a distinct allelic family of MSP2 sequences [20]. **Of the 41 isolates obtained in 1996, 40 were mono-clonal [12].
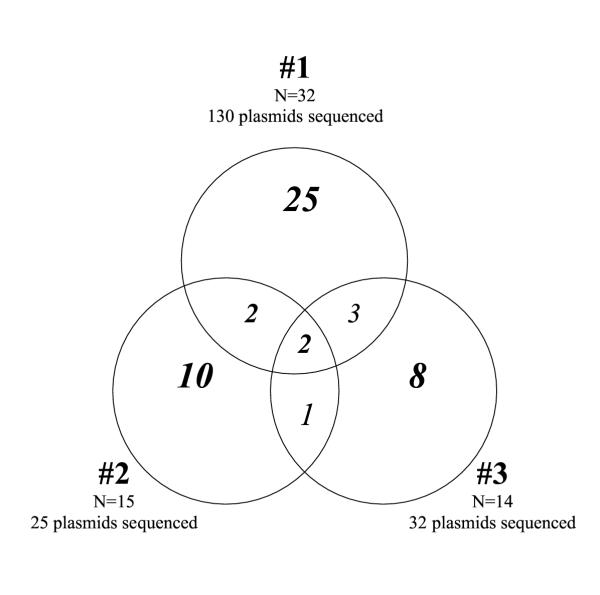
130 plasmids containing amplified dbl-α sequences encoding the DBL-1α domain of PfEMP1 from isolate #1 were sequenced. 32 distinct dbl-α sequences were obtained, 55% of the estimated full complement of genes [2,3]. Similarly, 15 distinct dbl-α sequences were determined from isolate #2 and 14 from isolate #3. As previously reported by Taylor et al. (4), binomial probability analysis revealed a low level of measurable primer bias (data not shown), but this is unlikely to have affected our findings.
The three isolates share few dbl-α sequences, and so have distinct var repertoires with little overlap (Figure 1). Estimates of the probability that the isolates have identical var repertoires were obtained from pair-wise comparisons as follows:
Figure 1.
Overlap in dbl-α repertoires among 3 sympatric parasite isolates. Venn diagram showing the number of var dbl-α sequences that overlap among the three isolates. N refers to the number of unique dbl-α sequences among the total number of sequenced plasmids from each isolate.
Isolate #1 and isolate #2: (4 successes from 15 trials) P = 0.0273
Isolate #1 and isolate #3: (5 successes from 14 trials) P = 0.0748
Are var sequences common to sympatric isolates globally conserved?
Unrelated P. falciparum parasites may be expected to have some var loci in common, as approximately 40% of loci are located in central chromosome regions [3] and, therefore, are less likely to diverge through sub-telomeric recombination [2,14]. In addition, recent work has demonstrated functional conservation of genes encoding placental-binding PfEMP1, and this conservation may be preserved by either chromosomal location or orientation with respect to the telomere [15]. Such functional conservation may also hold for other sub-classes of PfEMP1. Of the 51 distinct dbl-α sequences described herein, only eight were found in more than one of our three sympatric isolates. These eight sequences were used to search the annotated genome of P. falciparum clone 3D7 for similar var genes of known chromosomal location.
The closest 3D7 BLAST match for three of these eight sequences belonged to the Type 1 var class (which includes 38 of the 59 described 3D7 loci), but two of those were in central chromosome locations (Table 2). Of the remaining five matches, three are in the group of sub-telomeric var genes oriented for transcription towards the telomere, which is expected to hinder recombination at these loci [3,14]. Therefore, of the loci common to at least two of our sympatric isolates, five show a similarity to 3D7 var loci in chromosomal sites where ectopic recombination is not favoured.
Table 2.
3D7 loci with sequence similarity to var loci found in at least two of the three isolates described in this study. Sequences from isolates 1 or 2 which were found in at least one other isolate were compared to sequences in the genome of clone 3D7 using BLAST software at the PlasmoDb site. The number of times each sequence was identified in each isolate is shown in columns two to four. The 3D7 locus with the most similarity (i.e. lowest probability) is shown in each case.
| DBL sequence | Isolate | 3D7 Locus | Probabillity | var class | Orientation | ||
| #1 | #2 | #3 | |||||
| #1-01 | 3 | - | 1 | Chr 6: MAL6P1.316 | 5.0 × e-42 | Atypical Type 2 | Away from telomere |
| #1-02 | 2 | - | 1 | Chr 6: MAL6P1.316 | 7.0 × e-38 | Atypical Type 2 | Away from telomere |
| #1-05 | 3 | 1 | - | Chr 9: PFI1820 | 2.1 × e-37 | Atypical Type 3 | Towards telomere |
| #1-08 | 7 | 5 | 3 | Chr 11: PF11_0521 | 3.0 × e-48 | Atypical Type 9 | Towards telomere |
| #1-09 | 2 | - | 6 | Chr12: PFL0020 | 5.4 × e-38 | Type 1 | Away from telomere |
| #1-14 | 1 | 2 | - | Chr 8: PF08_0106 | 2.2 × e-37 | Type 1 | Central cluster |
| #1-22 | 19 | 1 | 3 | Chr 4: PFD1000 / PFD0995 | 7.1/7.4 × e-42 | Type 1 | Duplicated; central cluster |
| #2-01 | - | 3 | 8 | Chr 8: PF08_0141 | 4.6 × e-46 | Atypical Type 2 | Towards telomere |
Discussion
The divergence in var repertoires we have observed fits with theoretical predictions that immune selection of polymorphic immunodominant antigens such as PfEMP1 can structure a parasite population into discrete strains defined by their antigenic phenotypes [2,11]. The small amount of overlap observed among the three examined isolates is not inconsistent with this, as it is unlikely that the entire var genotype actively contributes to the Varotype, or repertoire of PfEMP1 phenotypes expressed in the course of a single infection [2]. As a result, immune selection would not act upon all members of the genomic var repertoire. In addition, genomic data indicate that numerous var gene fragments and pseudogenes are scattered around the telomeric regions of P. falciparum chromosomes [3]. These may be among the sequences we have identified and can account for a degree of overlap in var repertoires at the genomic level. Further, we have shown that five of the eight shared var loci have similarity to 3D7 var genes located in the central chromosomal regions or in reverse orientation with respect to the telomere, positions in which ectopic homologous recombination may not be favoured [3,14]. These may, therefore, be "globally conserved" var genes.
We have shown that isolates #1 and #2, which between them represent 75% of the parasite population studied, are identical at all single-copy polymorphic loci examined [12], but differ in their repertoires of two multi-gene familes: rif (rrm) [12] and var. In fact, the var repertoire of isolate #2 is as different from that of isolate #1 as is that of the third isolate examined, which has no single-copy polymorphic loci in common with either. Therefore, these sub-telomeric loci accumulate diversity more rapidly than other loci in this parasite population. This is the first such demonstration in natural isolates and supports the hypothesis that ectopic recombination among sub-telomeres in P. falciparum is a crucial source of diversity among multicopy genes such as var and rif which are located there [5,14]. In the parasite population studied self-fertilisation is predicted to be a common event [12], and so ectopic recombination during meiosis is a likely major source of variation among the progeny. The contribution, if any, of mitotic recombination events remains unclear [5,14].
The dbl-α and rif 3' UTR sequences we have examined, while sharing a sub-telomeric chromosomal location, may accumulate diversity by different mechanisms. It is known that novel dbl-α sequences are generated by intragenic recombination [5], presumably facilitated by substantial tracts of homologous sequence to be found around each sub-telomeric var locus, which permit heterologous meiotic chromosome exchange [14]. This mechanism will also contribute to recombination among rif loci on heterologous chromosomes. However, in addition, the rif 3' UTR RRM sequence we have used for "fingerprinting" contains a six base-pair repeat sequence which varies in number, giving the size polymorphisms we have measured [12]. Thus, new RRM configurations can arise by slippage in the number of repeats at particular loci, as is seen in other polymorphic P. falciparum genes such as msp1 and msp2. Such slippage is probably exacerbated by ectopic recombination, as rif loci with different numbers of repeats pair and undergo exchange.
Conclusions
We have shown that sympatric parasites share few var gene sequences, even when alleles at other polymorphic loci are identical, consistent with immune selection acting against overlap in PfEMP1 repertoires. Demonstration that these distinct repertoires are widespread in the parasite population and stable over time, particularly in the Padamo population where self-fertilisation is predicted to be common, would provide stronger support for this interpretation. In parasite populations where outcrossing is the norm, genetic recombination is expected to exert a strong reshuffling effect, and stable inheritance of var repertoires by self-fertilisation will be less likely. Whether immune selection working against repertoire overlap can maintain a structured parasite population under such conditions remains an open question.
Authors' contributions
AT collected the samples, participated in design of the study, carried out cloning and sequencing of PCR products and contributed significantly to writing the manuscript. RO carried out cloning and sequencing of PCR products, performed clustal alignments and collated the sequence data. GATT participated in the design of the study, provided logistic support and contributed significantly to writing the manuscript. CJS conceived the study, participated in its design and coordination, carried out cloning and sequencing of PCR products and drafted the manuscript. All authors read and approved the manuscript.
Acknowledgments
Acknowledgements
This work received financial support from The Wellcome Trust, project no. 056770/Z/99/Z. We thank Dr Hajo Grundmann for helpful discussions.
Contributor Information
Adriana Tami, Email: adriana.tami@lshtm.ac.uk.
Rosalynn Ord, Email: rosalynn.ord@lshtm.ac.uk.
Geoffrey AT Targett, Email: geoff.targett@lshtm.ac.uk.
Colin J Sutherland, Email: colin.sutherland@lshtm.ac.uk.
References
- Su X-Z, Heatwole VM, Wertheimer SP, Guinet F, Herrfeldt JA, Peterson DS, Ravetch JA, Wellems TE. A large and diverse gene family (var) encodes 250–300 kD proteins implicated in the antigenic variation and cytoadherence of Plasmodium falciparum-infected erythrocytes. Cell. 1995;82:89–100. doi: 10.1016/0092-8674(95)90055-1. [DOI] [PubMed] [Google Scholar]
- Sutherland CJ. The flip-side of cytoadherence: Immune selection, antigenic variation and the var genes of Plasmodium falciparum. Parasitol Today. 1998;14:329–332. doi: 10.1016/S0169-4758(98)01276-9. [DOI] [PubMed] [Google Scholar]
- Gardner MJ, Hall N, Fung E, White O, Berriman M, Hyman RW, Carlton JM, Pain A, Nelson KE, Bowman S, et al. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature. 2002;419:498–511. doi: 10.1038/nature01097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor HM, Kyes SA, Harris D, Kriek N, Newbold CI. A study of var gene transcription in vitro using universal var gene primers. Mol Biochem Parasitol. 2000;105:13–23. doi: 10.1016/S0166-6851(99)00159-0. [DOI] [PubMed] [Google Scholar]
- Taylor HM, Kyes SA, Newbold CI. Var gene diversity is generated by frequent recombination events. Mol Biochem Parasitol. 2000;110:391–397. doi: 10.1016/S0166-6851(00)00286-3. [DOI] [PubMed] [Google Scholar]
- Kirchgatter K, Mosbach R, del Portillo HA. Plasmodium falciparum : DBL-1 var sequence analysis in field isolates from Central Brazil. Exp Parasitol. 2000;95:154–157. doi: 10.1006/expr.2000.4520. [DOI] [PubMed] [Google Scholar]
- Fowler EV, Peters JM, Gatton ML, Chen N, Cheng Q. Genetic diversity of the DBLα region in P lasmodium falciparum var genes among Asia-Pacific isolates. Mol Biochem Parasitol. 2002;120:117–126. doi: 10.1016/S0166-6851(01)00443-1. [DOI] [PubMed] [Google Scholar]
- Aguir JC, Albrecht GR, Cegielski P, Greenwood BM, Jensen JB, Lallinger G, Martinez A, McGregor IA, Minjas JN, Neequaye J, Patarroyo ME, Sherwood JA, Howard RJ. Agglutination of Plasmodium falciparum-infected erythrocytes from East and West African isolates by human sera from distant geographic regions. Am J Trop Med Hyg. 1992;47:621–632. doi: 10.4269/ajtmh.1992.47.621. [DOI] [PubMed] [Google Scholar]
- Giha HA, Theander TG, Staalsoe , Roper C, Elhassan IM, Babiker H, Satti GMH, Arnot DE, Hviid L. Seasonal variation in agglutination of Plasmodium falciparum-infected erythrocytes. Am J Trop Med Hyg. 1998;58:399–405. doi: 10.4269/ajtmh.1998.58.399. [DOI] [PubMed] [Google Scholar]
- Bull PC, Lowe BS, Kortok M, Molyneux CS, Newbold CI, Marsh K. Parasite antigens on the infected red cell surface are targets for naturally acquired immunity to malaria. Nature Med. 1998;4:358–360. doi: 10.1038/nm0398-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Maiden MCJ, Feavers IM, Nee S, May RM, Anderson RM. The maintenance of strain structure in populations of recombining infectious agents. Nature Med. 1996;2:437–442. doi: 10.1038/nm0496-437. [DOI] [PubMed] [Google Scholar]
- Tami A, Grundmann H, Sutherland C, McBride JS, Cavanagh DR, Campos E, Snounou G, Barnabe C, Tibayrenc M, Warhurst DC. Restricted genetic and antigenic diversity of Plasmodium falciparum under mesoendemic transmission in the Venezuelan Amazon. Parasitology. 2002;124:569–581. doi: 10.1017/s0031182002001713. [DOI] [PubMed] [Google Scholar]
- Bland M. In: An Introduction to Medical Statistics. OUP Oxford. 1987;Chapter 6, sect 64 [Google Scholar]
- Freitas-Junior LH, Bottius E, Pirrit LA, Deitsch KW, Scheidig C, Guinet F, Nehrbass U, Wellems TE, Scherf A. Frequent ectopic recombination of virulence factor genes in telomeric chromosome clusters of P. falciparum. Nature. 2000;407:1018–1022. doi: 10.1038/35039531. [DOI] [PubMed] [Google Scholar]
- Vazquez-Macias A, Martinez-Cruz P, Castaneda-Patian MC, Scheidig C, Gysin J, Scherf A, Hernandez-Rivas R. A distinct 5' flanking var gene region regulates Plasmodium falciparum variant erythrocyte surface antigen expression in placental malaria. Mol Microbiol. 2002;45:155–167. doi: 10.1046/j.1365-2958.2002.02999.x. [DOI] [PubMed] [Google Scholar]