Abstract
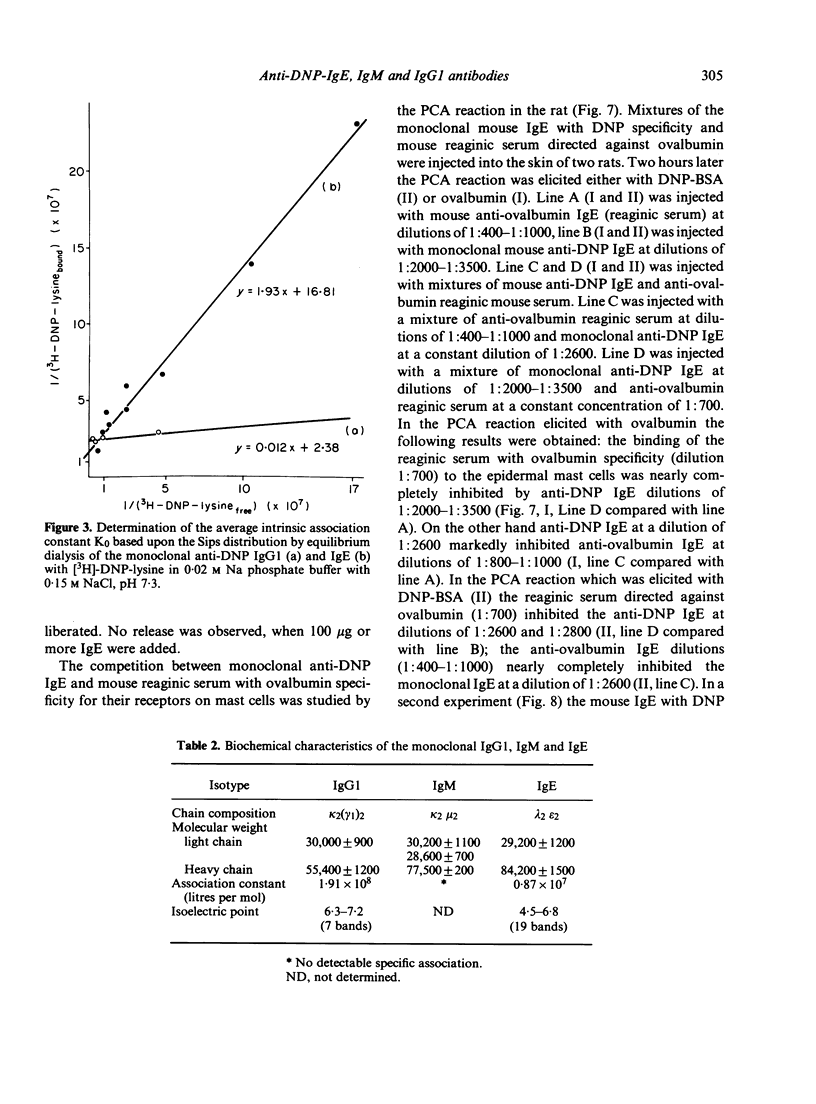
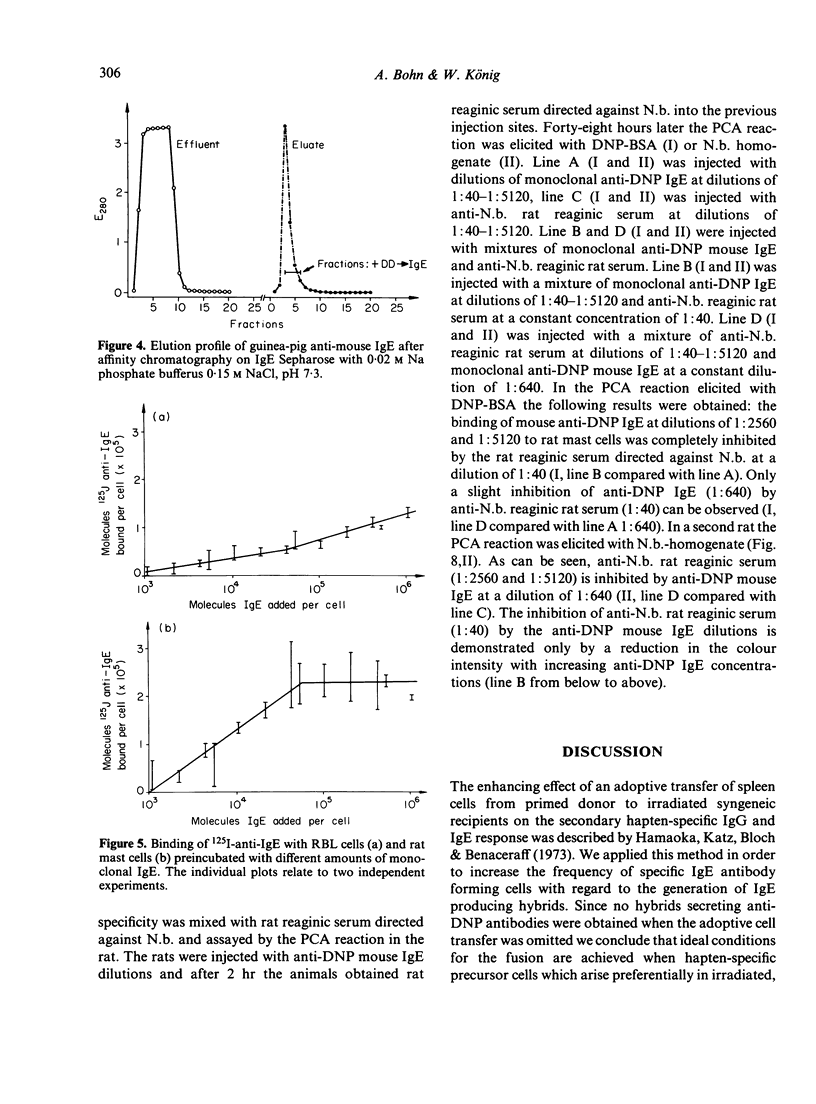
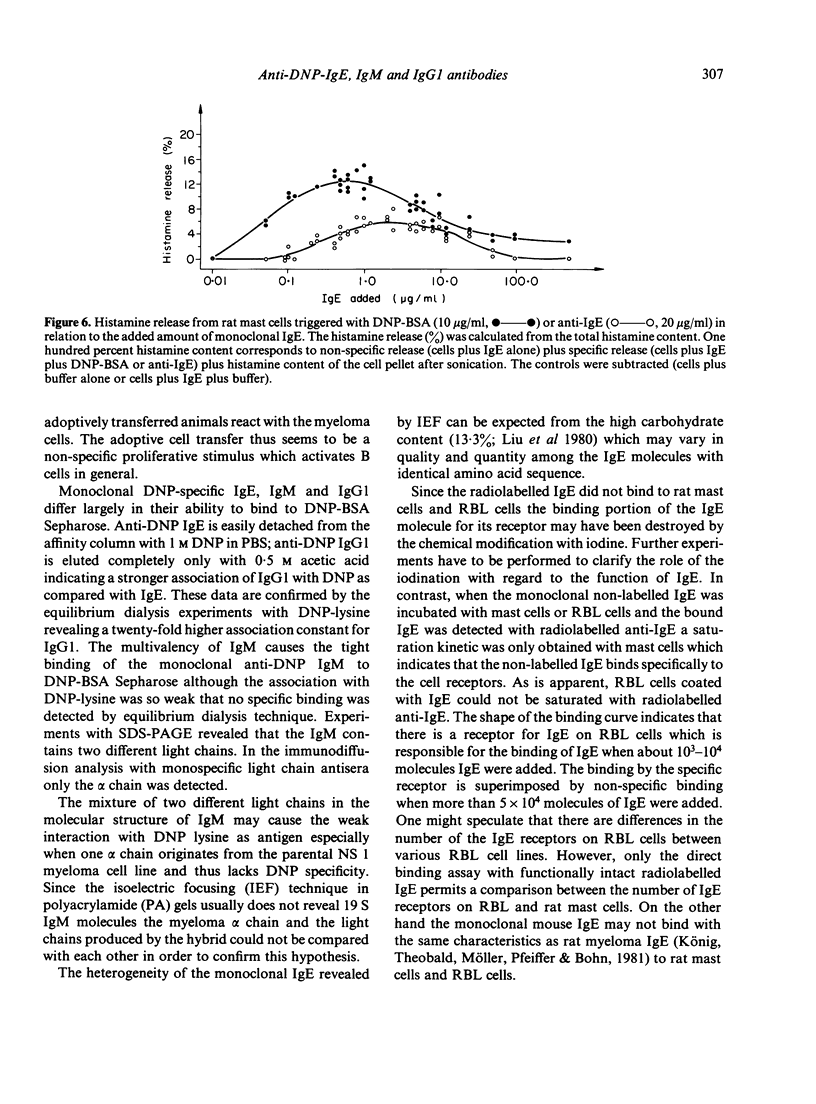
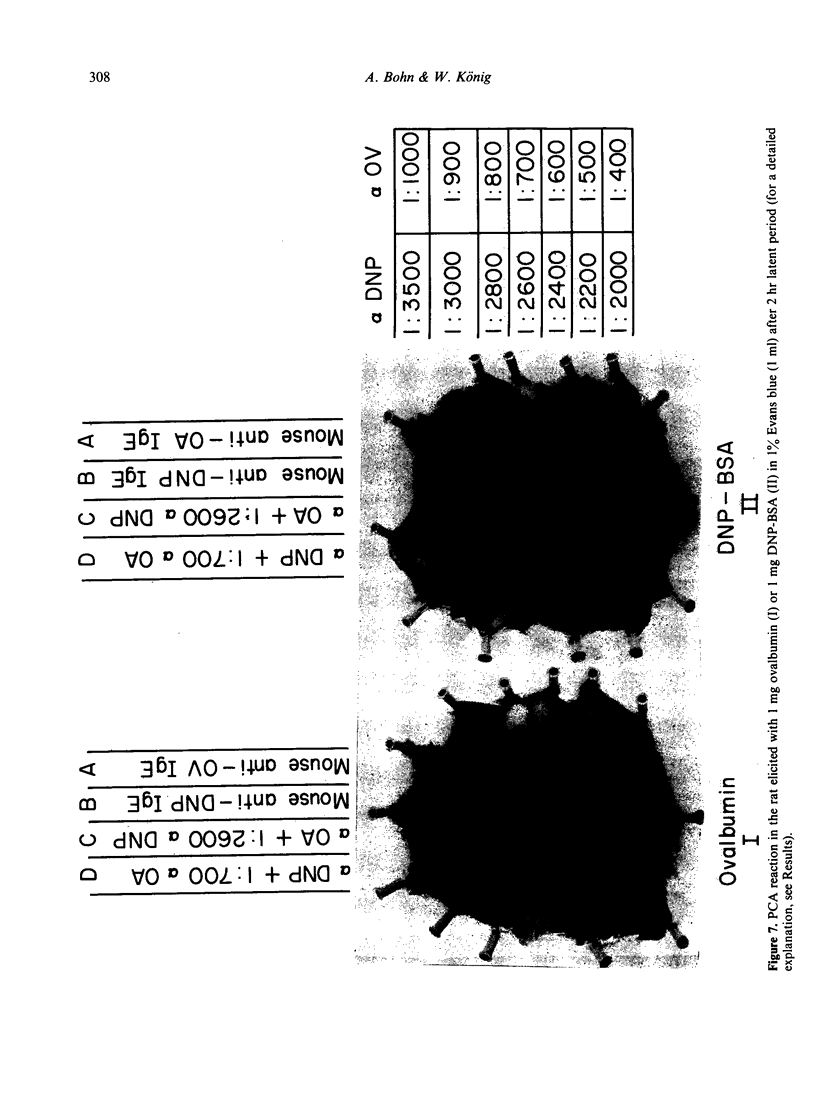
Monoclonal DNP-specific IgG (lambda 2 epsilon 2), IgM (kappa 2 mu2) and IgG [kappa 2 (gamma 1)2] were isolated fom the culture supernatant of hybridomas by affinity chromatography with 2,4-dinitrophenol bovine serum albumin (DNP-BSA) sepharose and characterized by biochemical and biological methods. The molecular weights were 84,200 for the epsilon chain, 55,400 for the gamma chain and 77,500 for the mu chain as determined by sodium dodecylsulphate polyacrylamide del electrophoresis (SDS-PAGE). The association constants for [3H]-DNP-lysine determined by equilibrium dialysis were 0 . 87 X 10(7) l/mol for IgE and 1 . 91 X 10(8) 1/mol for IgG1. The isoelectric focusing of the purified monoclonal antibodies revealed for IgG1 seven bands at a pH range of 6 . 3 - 7 . 2 and for IgE sixteen bands at a pH range of 4 . 5 to 6 . 8. the binding of 125I-anti-IgE to rat basophilic leukaemia (RBL) and rat mast cells which had been preincubated with various amounts of monoclonal IgE was studied. At saturation conditions of IgE, about 2 . 14 X 10(5) molecules of anti-IgE were bound per rat mast cell. Rat mast cells coated with monoclonal anti-DNP IgE were triggered for the release of histamine in the presence of either the antigen or guinea-pig anti-mouse IgE. A mutual inhibition of the passive cutaneous anaphylaxis (PCA) reaction in the rat by either mixing mouse reaginic serum directed against ovalbumin or rat reaginic serum directed against Nippostrongylus brasiliensis with monoclonal mouse anti-DNP IgE was demonstrated.
Full text
PDF




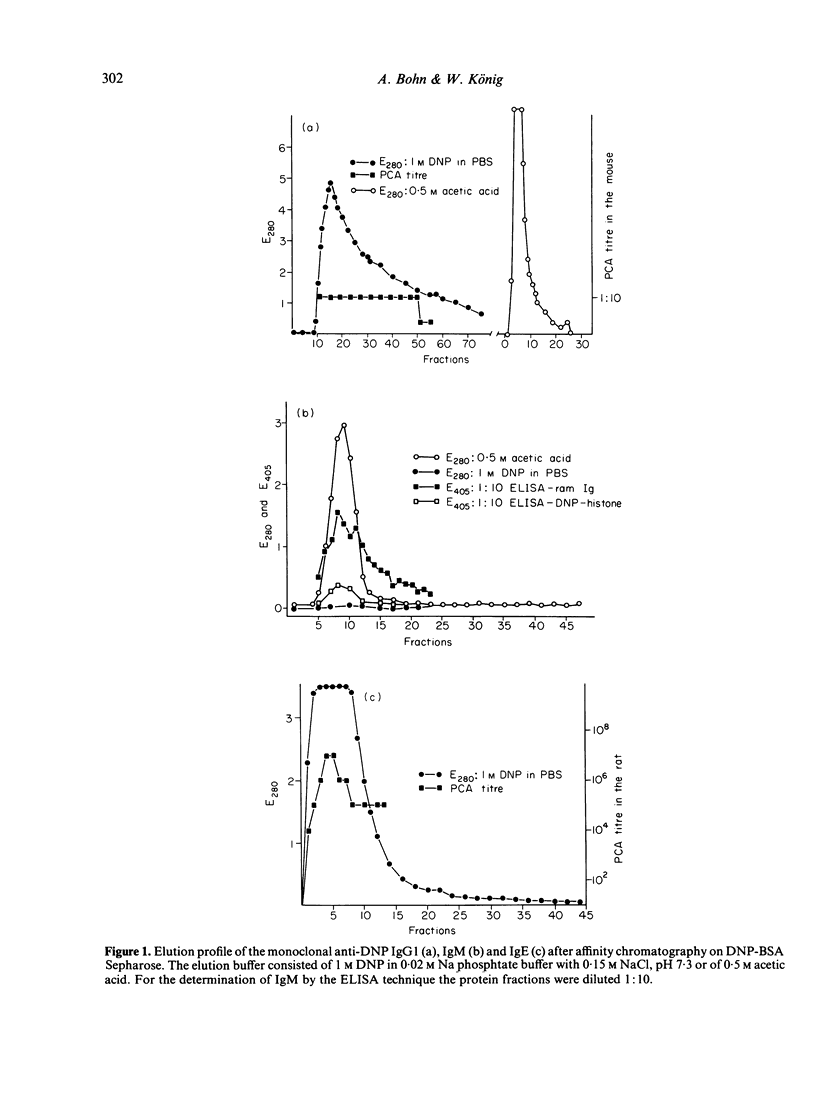
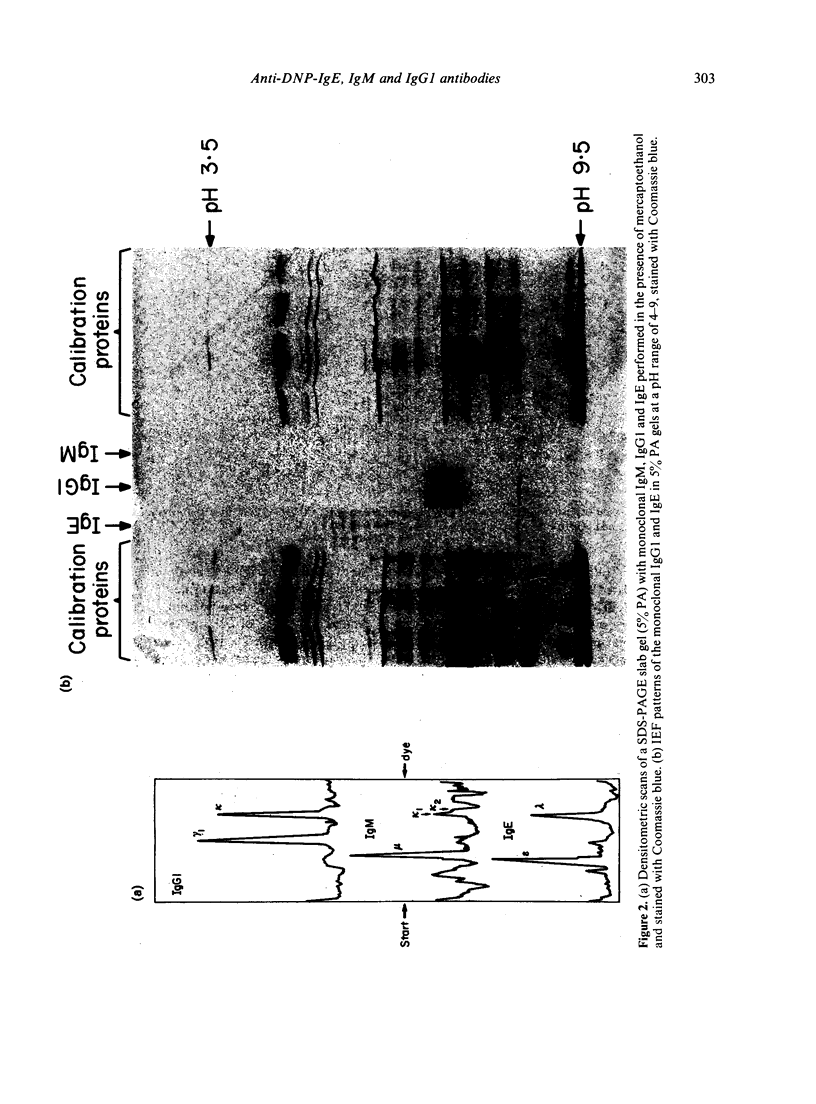
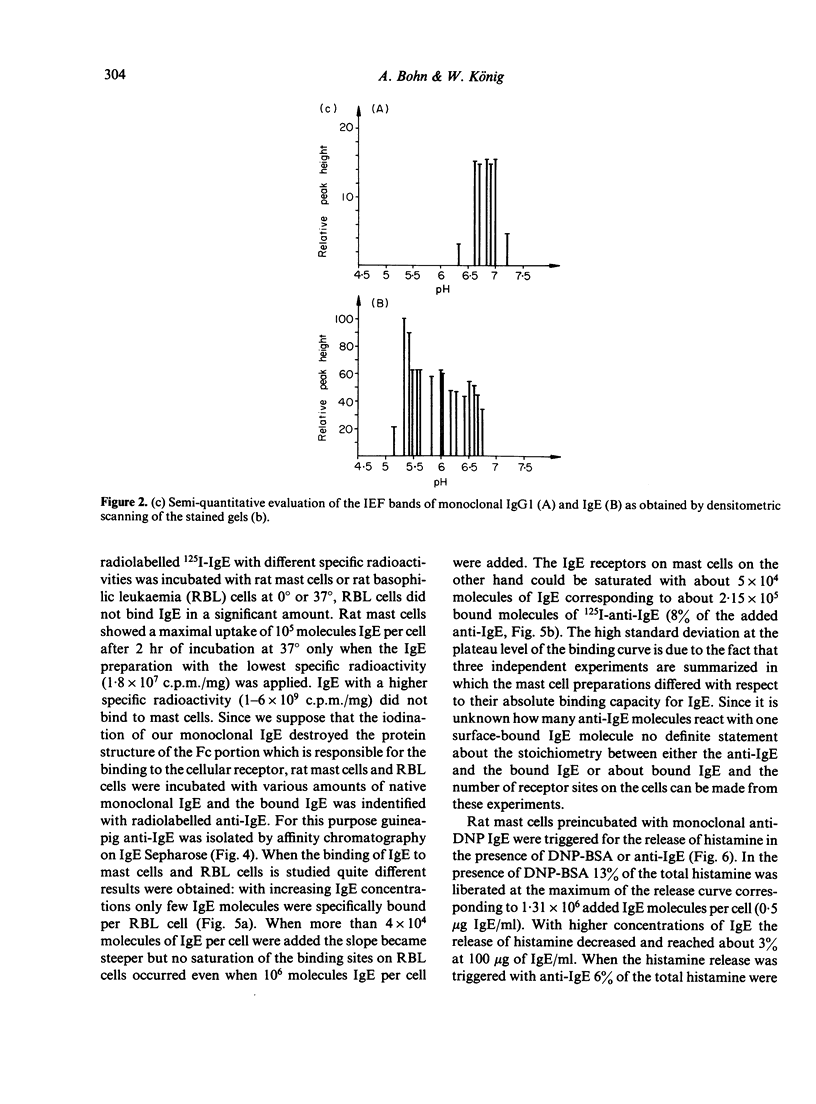


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bohn A., König W. Generation of monoclonal DNP-specific IgM and IgE murine antibodies on the efficacy of hybridization. Mol Immunol. 1982 Feb;19(2):193–199. doi: 10.1016/0161-5890(82)90332-7. [DOI] [PubMed] [Google Scholar]
- Böttcher I., Hämmerling G. Continuous production of monoclonal mouse IgE antibodies with known allergenic specificity by a hybrid cell line. Nature. 1978 Oct 26;275(5682):761–762. doi: 10.1038/275761a0. [DOI] [PubMed] [Google Scholar]
- David G. S., Reisfeld R. A. Protein iodination with solid state lactoperoxidase. Biochemistry. 1974 Feb 26;13(5):1014–1021. doi: 10.1021/bi00702a028. [DOI] [PubMed] [Google Scholar]
- Eshhar Z., Ofarim M., Waks T. Generation of hybridomas secreting murine reaginic antibodies of anti-DNP specificity. J Immunol. 1980 Feb;124(2):775–780. [PubMed] [Google Scholar]
- Foreman J. C., Hallett M. B., Mongar J. L. The relationship between histamine secretion and 45calcium uptake by mast cells. J Physiol. 1977 Sep;271(1):193–214. doi: 10.1113/jphysiol.1977.sp011996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaoka T., Katz D. H., Bloch K. J., Benacerraf B. Hapten-specific IgE antibody responses in mice. I. Secondary IgE responses in irradiated recipients of syngeneic primed spleen cells. J Exp Med. 1973 Jul 1;138(1):306–311. doi: 10.1084/jem.138.1.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulczycki A., Jr, Metzger H. The interaction of IgE with rat basophilic leukemia cells. II. Quantitative aspects of the binding reaction. J Exp Med. 1974 Dec 1;140(6):1676–1695. doi: 10.1084/jem.140.6.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Derivation of specific antibody-producing tissue culture and tumor lines by cell fusion. Eur J Immunol. 1976 Jul;6(7):511–519. doi: 10.1002/eji.1830060713. [DOI] [PubMed] [Google Scholar]
- Liu F. T., Bohn J. W., Ferry E. L., Yamamoto H., Molinaro C. A., Sherman L. A., Klinman N. R., Katz D. H. Monoclonal dinitrophenyl-specific murine IgE antibody: preparation, isolation, and characterization. J Immunol. 1980 Jun;124(6):2728–2737. [PubMed] [Google Scholar]
- March S. C., Parikh I., Cuatrecasas P. A simplified method for cyanogen bromide activation of agarose for affinity chromatography. Anal Biochem. 1974 Jul;60(1):149–152. doi: 10.1016/0003-2697(74)90139-0. [DOI] [PubMed] [Google Scholar]
- Morrison M. Lactoperoxidase-catalyzed iodination as a tool for investigation of proteins. Methods Enzymol. 1980;70(A):214–220. doi: 10.1016/s0076-6879(80)70051-4. [DOI] [PubMed] [Google Scholar]
- Möller G., König W. Binding characteristics of aggregated IgGa to rat basophilic leukaemia (RBL) cells and rat mast cells. Immunology. 1980 Nov;41(3):605–615. [PMC free article] [PubMed] [Google Scholar]
- Rudolph A. K., Burrows P. D., Wabl M. R. Thirteen hybridomas secreting hapten-specific immunoglobulin E from mice with Iga or Igb heavy chain haplotype. Eur J Immunol. 1981 Jun;11(6):527–529. doi: 10.1002/eji.1830110617. [DOI] [PubMed] [Google Scholar]
- Segal D. M., Sharrow S. O., Jones J. F., Siraganian R. P. Fc (IgG) receptors on rat basophilic leukemia cells. J Immunol. 1981 Jan;126(1):138–145. [PubMed] [Google Scholar]
- Siraganian R. P. An automated continuous-flow system for the extraction and fluorometric analysis of histamine. Anal Biochem. 1974 Feb;57(2):383–394. doi: 10.1016/0003-2697(74)90093-1. [DOI] [PubMed] [Google Scholar]