Abstract
Background
The SF-6D is a new single summary preference-based measure of health derived from the SF-36. Empirical work is required to determine what is the smallest change in SF-6D scores that can be regarded as important and meaningful for health professionals, patients and other stakeholders.
Objectives
To use anchor-based methods to determine the minimally important difference (MID) for the SF-6D for various datasets.
Methods
All responders to the original SF-36 questionnaire can be assigned an SF-6D score provided the 11 items used in the SF-6D have been completed. The SF-6D can be regarded as a continuous outcome scored on a 0.29 to 1.00 scale, with 1.00 indicating "full health".
Anchor-based methods examine the relationship between an health-related quality of life (HRQoL) measure and an independent measure (or anchor) to elucidate the meaning of a particular degree of change. One anchor-based approach uses an estimate of the MID, the difference in the QoL scale corresponding to a self-reported small but important change on a global scale. Patients were followed for a period of time, then asked, using question 2 of the SF-36 as our global rating scale, (which is not part of the SF-6D), if there general health is much better (5), somewhat better (4), stayed the same (3), somewhat worse (2) or much worse (1) compared to the last time they were assessed. We considered patients whose global rating score was 4 or 2 as having experienced some change equivalent to the MID. In patients who reported a worsening of health (global change of 1 or 2) the sign of the change in the SF-6D score was reversed (i.e. multiplied by minus one). The MID was then taken as the mean change on the SF-6D scale of the patients who scored (2 or 4).
Results
This paper describes the MID for the SF-6D from seven longitudinal studies that had previously used the SF-36.
Conclusions
From the seven reviewed studies (with nine patient groups) the MID for the SF-6D ranged from 0.010 to 0.048, with a weighted mean estimate of 0.033 (95% CI: 0.029 to 0.037). The corresponding Standardised Response Means (SRMs) ranged from 0.11 to 0.48, with a mean of 0.30 and were mainly in the "small to moderate" range using Cohen's criteria, supporting the MID results. Using the half-standard deviation (of change) approach the mean effect size was 0.051 (range 0.033 to 0.066). Further empirical work is required to see whether or not this holds true for other patient groups and populations.
Introduction
Health Related Quality of Life (HRQoL) outcome measures are being increasingly used in research trials, but less so in routine clinical practice. The interpretation of HRQoL scores raises many issues. [1-7] The scales and instruments used may be unfamiliar to many clinicians and patients, who may be uncertain of the meaning of the scale values and summary scores. [8]
Repeated experience and familiarity with a wide variety of physiological measures such as blood pressure or forced expiratory volume, has allowed clinicians to make meaningful interpretation of the results. [9,10] In contrast, the meaning of a change in score of x points on a HRQoL instrument is less intuitively apparent, not only because the scale has unfamiliar units, but also because health professionals seldom use HRQoL measures in routine clinical practice.
In clinical trials, where HRQoL instruments are being increasingly used as primary outcome measures, it is simple to determine the statistical significance of a change in HRQoL, but placing the magnitude of these changes in a context that is meaningful for health professionals, patients and other stakeholders (Pharmaceutical and Medical Device Developers, Insurance Payers, Regulators, Governments) has not been so easy. Ascertaining the magnitude of change that corresponds to a minimal important difference would help address this problem. [11] So when determining an important change standard the perspective can influence the assessment approach and the way in which an important difference is determined. [5] The minimal important difference (MID), from the patient perspective, can be defined as "the smallest difference in score in the domain of interest which patients perceive as beneficial and which would mandate, in the absence of troublesome side effects and excessive cost, a change in the patient's management". [9]
Thus, individual change standards are needed to provide meaningful interpretation of HRQoL intervention and treatment effects and to classify patients based on this standard as improved, stable or declined. To date two broad strategies have been used to interpret differences or changes in HRQoL following treatment: [12] distribution based approaches – the effect size (ES); and anchor-based measures – the minimum clinically important difference (MCID).
Distribution based approaches rely on relating the difference between treatment and control groups to some measure of variability. The most popular approach uses Cohen's [13] standardised effect size, the mean change divided by the standard deviation to serve as an "effect size index", that is suitable for sample size estimation. Cohen suggested that standardised effect sizes of 0.2 to 0.5 should be regarded as "small", 0.5 to 0.8 as "moderate" and those above 0.8 as "large". Cohen's effect size may be influenced by the degree of homogeneity or heterogeneity in the sample. Distribution-based methods rely on expressing an effect in terms of the underlying distribution of the results. Investigators may express effects in terms of between-person standard deviation units, within-person standard deviation units, and the standard error of measurement. [2]
Four statistics commonly used to index responsiveness are: [14]
1. effect size; [15]
2. t-test comparisons; [16]
3. the standardised response mean; [17]
4. the responsiveness statistic. [18]
The formula for these statistics are as follows, where D = raw score change on measure; SE = standard error of the difference; SD = standard deviation at time 1; SD* = standard deviation of D; SD# = standard deviation of D among stable subjects (those who true status is constant over time):
Paired t-statistics = D/SE
Effect size (ES) statistic = D/SD
Standardised response mean (SRM) = D/SD*
Responsiveness statistic = D/SD#
The paired t-statistic is best suited to pre-post assessments of interventions of known efficacy. The effect size statistic relates change over time to the standard deviation of baseline scores. The standardised response mean compares change to the standard deviation of change. The responsiveness statistic looks at HRQoL change relative to variability for clinically stable respondents. The effect size statistic ignores variation in change entirely, the t-statistic ignores information about variation in scores for clinically stable respondents, and the responsiveness statistic ignores information about variation in scores for clinically unstable responders.
Anchor-based methods examine the relationship between an HRQoL measure and an independent measure (or anchor) to elucidate the meaning of a particular degree of change. Thus anchor-based approaches require an independent standard or anchor that is itself interpretable and at least moderately correlated with the instrument being explored. [2] One anchor-based approach uses an estimate of the MID, the difference on the HRQoL scale corresponding to self-reported small but important change on a global scale.[9]
Norman et al mention several problems with the global assessment of change including, that the reliability and validity of the global scale has not been established and that the judgement of change is psychologically difficult. [19] Another limitation of the global rating is that is does not represent a criterion or gold standard for assessment of change and yet we use the global rating as an anchor to define small, medium and large changes. [9,11]
No single approach to interpretability is perfect. As Guyatt et al suggest the use of multiple strategies is likely to enhance the interpretability of any particular instrument. [2] Therefore we used both distribution and anchor-based approaches to try and establish the interpretability of the SF-6D, a new single summary preference-based measure of health derived from the SF-36.
The SF-36 is one of the most widely used HRQoL outcome measures in the world today. It contains 36 questions measuring health across eight dimensions – physical functioning, role limitation because of physical health, social functioning, vitality, bodily pain, mental health, role limitation because of emotional problems and general health. Responses to each question within a dimension are combined to generate a score from 0 to 100, where 100 indicates "good health". [20] Thus, the SF-36 generates a profile of HRQoL outcomes (on up to eight dimensions), which makes statistical analysis and interpretation difficult. [8]
The developers of the SF-36 have suggested that using the general health dimension a five-point difference (on the 0–100 scale) is the smallest score change achievable by an individual and considered as 'clinically and socially relevant'. [21] Angst et al found the MCID ranged from 3.3 to 5.3 points on the physical function dimension and 7.2 to 7.8 points on the bodily pain dimension in patients with osteoarthritis of the hip or knee. [22] Hays and Morales also provide information on what a clinically important difference is for the SF-36 scales. They conclude that the MCID for the SF-36 is "typically in the range of 3–5 points", although they also recommend caution in interpreting 3–5 points on the SF-36 dimensions as the MCID. [23]
The method of scoring the SF-36 is not based on preferences. The simple scoring algorithm for the eight dimensions assumes equal intervals between the response choices, and that all items are of equal importance, which may not be appropriate. The SF-6D is a new single summary preference based or utility measure of health derived from the SF36. [24,25] Empirical work is required to determine what is the smallest change in SF-6D scores that can be regarded as important. We used anchor-based methods to determine the MID for the SF-6D for various datasets.
Methods
The Questionnaire: SF-6D Health State Classification
The SF-36 was revised into a six-dimensional health state classification called the SF-6D. The six dimensions are physical functioning, role limitations, social functioning, pain, mental health and vitality. These six dimensions each have between two and six levels. An SF-6D "health state" is defined by selecting one level from each dimension. A total of 18,000 health states are thus defined. All responders to the original SF-36 questionnaire can be assigned SF-6D score provided the 11 items used in the six dimensions of the SF-6D have been completed. The SF-6D preference-based measure can be regarded as a continuous outcome scored on a 0.29 to 1.00 scale, with 1.00 indicating "full health". [24,25]
The studies
The data used in this paper comes from seven longitudinal studies and (nine patient groups), which used the SF-36 including randomised controlled trials, [26] and observational studies. [27-30]
Global Rating of change (GRoC)
Patients were followed for a period of time, then asked, using question 2 of the SF-36 as our global rating of change scale, (which is not part of the SF-6D), if:
2. Compared to one year ago, how would you rate your health in general now?
(5) Much better now than one year ago
(4) Somewhat better now than one year ago.
(3) About the same
(2) Somewhat worse now than one year ago.
(1) Much worse now than one year ago
The original question 2 of the SF36 compares health now with one year ago. Depending on the follow-up time we used a slightly modified version: e.g. health now compared to three (or six) months ago.
Statistical Analysis
We examined the relationship between the global ratings of change question and changes in SF-6D score, by calculating the change in SF-6D score from 1st to 2nd assessment for each patient. We considered patients whose GRoC score was 4 or 2 as having experienced some change equivalent to the MID. In patients who reported a worsening of health (GRoC of 1 or 2) the sign of the change in the SF-6D score was reversed (i.e. multiplied by minus one). The MID was then taken as the mean change on the SF-6D scale of the patients who scored (2 or 4).
Since the SF-6D is a continuous measure of effect we used meta-analytic methods to estimate the weighted grand mean of the MID and to test the hypothesis of homogeneity of MID across the nine studies. If there was no statistical evidence of lack of homogeneity, a 95% confidence interval for the summary estimate of the MID was then calculated. [31,32]
We also used a distribution-based approach and calculated a standardised response mean (SRM). Since the standard error of the SRM is not defined we used bootstrap methods to estimate 95% confidence intervals for the SRM. [33]
Global measures of change are typically highly correlated with the present state and uncorrelated with the initial state. Any measure of change that reflects the unbiased difference between the final and initial state, should show a positive correlation with the final state and an equal negative correlation with the initial state. [19] We therefore also calculated Pearson's product moment correlations between the GRoC question and the baseline and follow-up SF-6D scores.
Results
This paper describes the MID for the SF-6D from a variety of longitudinal studies, with different patient groups and length of follow-up that had previously used the SF-36 (Table 1).
Table 1.
The nine longitudinal studies
| Study/patient group | Total study size | Number who reported some change | Period of time |
| Older adults (aged >65 years): 1st follow-up | 4945 | 1362 | Baseline to year 1 |
| Older adults (aged >65 years): 2nd follow-up | 3127 | 948 | Year 1 to year 2 |
| Irritable bowel syndrome (IBS) patients | 137 | 56 | Baseline to 3 months |
| Irritable bowel syndrome (IBS) control patients | 177 | 27 | Baseline to 3 months |
| Leg ulcer patients | 194 | 45 | Baseline to 3 months |
| Knee Osteoarthritis (OA) patients | 157 | 59 | Baseline to 6 months |
| Limb reconstruction patients | 60 | 29 | Baseline to year 1 |
| Early Rheumatoid Arthritis (RA) patients | 246 | 99 | Baseline to year 1 |
| Patients with Chronic Obstructive Pulmonary Disease (COPD) | 60 | 29 | Baseline to year 1 |
Total study size = no. of patients with valid baseline and follow-up SF-6D score and follow-up global change score.
Table 2 shows that from the nine patient groups the MID for the SF-6D ranged from 0.010 to 0.048, with a mean 0.030 and a median 0.032. The wide confidence intervals for the MID estimates, including negative values, reflect both the uncertainty in the estimates and the small study sizes. The corresponding effect sizes (SRMs) ranged from 0.11 to 0.48, mean 0.30 and were mainly in the "small to moderate" range using Cohen's criteria. Using a half-standard deviation of change approach the mean effect size was 0.051 and ranged from 0.033 to 0.066. This suggests that the results obtained through the MID method are reasonable and generally of similar size to the effect size (SRM) estimates. It demonstrates that regardless of the method used, the actual cut-off point for a clinically important difference is going to be in the same neighbourhood, thereby making the particular method of approach less important.
Table 2.
Minimum Important Difference (MID's) and Effect Sizes (SRM's)
| Study/patient group | N | MID – Mean change in SF-6D (95% CI)* | Standard Deviation | Effect Size (SRM) (95% CI)* | 0.5 of a Standard Deviation |
| Older adults 1st follow-up | 1362 | 0.039 (0.034 to 0.044) | 0.099 | 0.39 (0.35 to 0.45) | 0.050 |
| Older adults 2nd follow-up | 948 | 0.026 (0.021 to 0.033) | 0.096 | 0.27 (0.22 to 0.34) | 0.048 |
| IBS patients | 56 | 0.023 (-0.001 to 0.050) | 0.096 | 0.24 (-0.03 to 0.52) | 0.048 |
| IBS control patients | 27 | 0.025 (-0.013 to 0.071) | 0.113 | 0.22 (-0.15 to 0.62) | 0.057 |
| Leg ulcer patients | 45 | 0.032 (-0.001 to 0.071) | 0.131 | 0.24 (-0.06 to 0.53) | 0.066 |
| OA Knee patients | 59 | 0.032 (0.015 to 0.049) | 0.066 | 0.49 (0.22 to 0.73) | 0.033 |
| Limb reconstruction patients | 29 | 0.048 (0.007 to 0.091) | 0.120 | 0.40 (-0.02 to 0.79) | 0.060 |
| Early (RA) patients | 99 | 0.039 (0.017 to 0.061) | 0.112 | 0.33 (0.14 to 0.58) | 0.056 |
| COPD Patients | 29 | 0.010 (-0.019 to 0.043) | 0.087 | 0.11 (-0.28 to 0.47) | 0.044 |
*Bootstrap Bias-Corrected and accelerated (BCA) 95% Confidence Intervals.
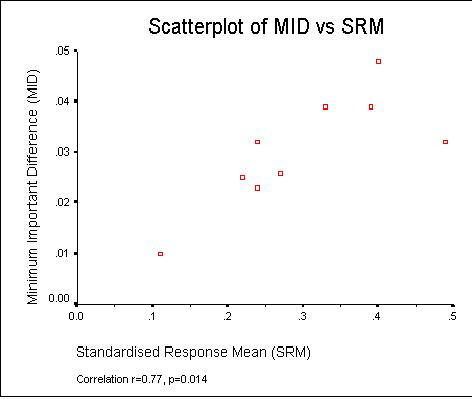
As expected since the MID and SRM both contain the mean change, Figure 2 shows there was a strong correlation (r = 0.70, p = 0.014) between the MID and SRM estimates (see Figure 1). There was no reliable evidence of an association between the MID and the time between assessments (correlation r = 0.24, p = 0.54) in our nine studies.
Figure 2.
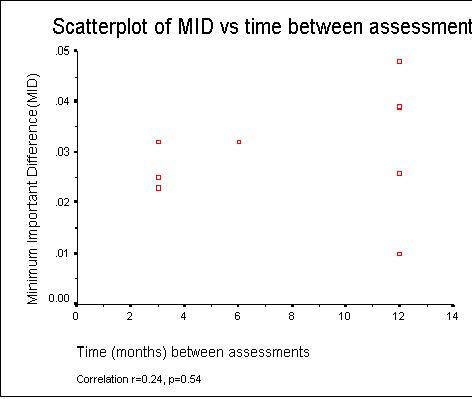
Figure 1.
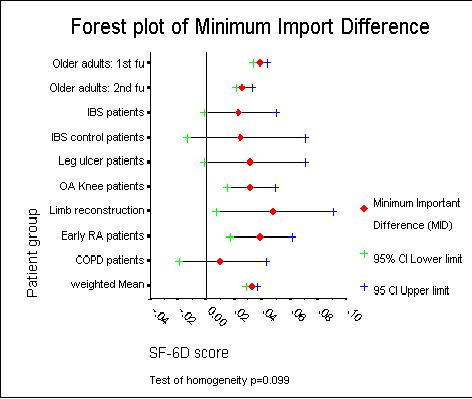
There was no reliable statistical evidence of lack of homogeneity in the MID estimates across the nine studies (χ2 = 13.41 on 8 df, p = 0.098). Therefore it seemed reasonable to combine the MID estimates from the nine studies to produce an overall weighted grand mean MID estimate of 0.033 (95% CI: 0.029 to 0.037). Figure 3 shows a forest plot of the MID estimates and associated confidence limits for the nine studies and the estimated combined overall weighted grand mean MID.
Figure 3.
The combining of the "somewhat worse" and "somewhat better" groups assumes the two cohorts are identical except for the sign. Table 3 suggests some evidence that the magnitude of the MID for those who improved and those whose deteriorated is different, but this result was not statistically significant.
Table 3.
Magnitude of the MID by worse/better
| Global rating of health change | ||||||
| Somewhat worse | Somewhat better | |||||
| Study/patient group | N | Mean change (SD) | N | Mean change (SD) | Mean Difference (95% CI) | P-value |
| Older adults 1st follow-up | 1087 | 0.039 (0.099) | 275 | 0.042 (0.098) | -0.004 (-0.017 to 0.009) | 0.58 |
| Older adults 2nd follow-up | 783 | 0.028 (0.095) | 165 | 0.019 (0.102) | 0.009 (-0.008 to 0.026) | 0.32 |
| IBS patients | 36 | 0.022 (0.096) | 20 | 0.026 (0.097) | -0.040 (-0.059 to 0.051) | 0.87 |
| IBS control patients | 15 | 0.017 (0.130) | 12 | 0.035 (0.094) | -0.018 (-0.107 to 0.071) | 0.69 |
| Leg ulcer patients | 14 | 0.082 (0.109) | 31 | 0.009 (0.105) | 0.073 (-0.03 to 0.176) | 0.08 |
| OA Knee patients | 30 | 0.003 (0.073) | 29 | 0.036 (0.059) | -0.007 (-0.041 to 0.028) | 0.70 |
| Limb reconstruction patients | 10 | 0.044 (0.14) | 19 | 0.051 (0.112) | -0.007 (-0.117 to 0.102) | 0.88 |
| Early (RA) patients | 17 | -0.007 (0.117) | 82 | 0.046 (0.109) | -0.053 (-0.117 to 0.011) | 0.08 |
| COPD Patients | 19 | 0.012 (0.095) | 10 | 0.006 (0.074) | 0.006 (-0.061 to 0.072) | 0.87 |
P-value from two-independent samples t-test.
Table 4 shows the moderate correlations (mean 0.45, range: 0.18 to 0.57) were found between response to global change (anchor) GRoC question and the SF-6D at follow-up across the 9 studies. Lower correlations (mean 0.22, range: 0.01 to 0.41) were found between the response to the GRoC question and the SF-6D score at initial assessment across the nine studies.
Table 4.
Correlations between global health change scale and baseline and follow-up SF-6D scores.
| Baseline | Follow-up | ||||
| Study/patient group | N | r | p-value | r | p-value |
| Older adults (aged >65 years): 1st follow-up | 4945 | 0.324 | 0.001 | 0.390 | 0.001 |
| Older adults (aged >65 years): 2nd follow-up | 3127 | 0.395 | 0.001 | 0.514 | 0.001 |
| Irritable bowel syndrome (IBS) patients | 137 | 0.311 | 0.001 | 0.441 | 0.001 |
| Irritable bowel syndrome (IBS) control patients | 177 | 0.094 | 0.215 | 0.202 | 0.007 |
| Leg ulcer patients | 194 | 0.041 | 0.572 | 0.309 | 0.001 |
| Knee Osteoarthritis (OA) patients | 157 | 0.300 | 0.001 | 0.559 | 0.001 |
| Limb reconstruction patients | 60 | -0.007 | 0.959 | 0.458 | 0.001 |
| Early Rheumatoid Arthritis (RA) patients | 246 | 0.147 | 0.021 | 0.524 | 0.001 |
| Patients with Chronic Obstructive Pulmonary Disease (COPD) | 60 | 0.391 | 0.002 | 0.547 | 0.001 |
r = Pearsons Correlation Coefficient
Discussion
We used a five-point GRoC scale; others have used seven or 14 points, which may be more sensitive. [9,11] Although the designation of what GRoC suggests patients as fundamentally unchanged and what GRoC suggests patients have experienced a small but important change is inevitably subjective.
The reliability and validity of a single GRoC question has not been established. Multi-item scales may have better reliability. Indeed if the single GRoC could be shown to have superior measurement properties, then there is no reason not to simply use this a measure of HRQoL. Although Wyrwich found moderate-to-substantial agreement between the responses to question 2 of the SF-36 (weighted Kappa 0.64 – 0.73) at test and re-test (1–4 days later) in a group of 241 patients with asthma, coronary artery disease, congestive heart failure and COPD. This result provides some evidence of the usefulness of retrospective GRoC as patient-perceived anchors for ascertaining important HRQoL changes. [34]
The judgement of change is psychologically difficult. Patients must be able to quantify both their present state and their initial state and then perform a mental subtraction. Patients may be unable to recall their initial state, and the judgement is based on their present state and working backwards. Any measure of change that reflects the unbiased difference between the final and initial state, should show a positive correlation with the final state and an equal negative correlation with the initial state. [19] Our results found larger correlations between the global measures of change with the present state (HRQoL) and far lower correlations with the initial state, supporting this hypothesis.
The length of time between 1st and 2nd assessments was up to a year, which is far larger than the timeframes used in other studies (e.g. 2 weeks for Jaeschke et al [9] and 4 weeks for Juniper et al [11]). This may be a limitation of this study in the evaluation of clinical change, as patients may have some difficulty recalling their previous state of health. However, we found no reliable evidence of an association between the MID and the time between assessments in our nine studies. Although preliminary results with two older adults cohorts suggest some form of 'Response shift' and that the MID may not be constant over time.
We combined the worse and better groups into one and assumed that the magnitude of the MID for these two cohorts were identical except for the sign. We found no reliable statistical evidence that the magnitude of the MID for those who improved and deteriorated was different. This may be explained by the small sample sizes for some of the studies and the low power to detect anything other than large differences in the mean changes. Thus the small sample sizes can explain the lack of statistical significance of the difference in MID between the worse and better groups, but not the overall size of the observed mean difference, which for some studies was more than twice as great in one group compared to the other.
We used a single anchor; our results require validation with alternative anchors or multiple anchor methods. Other approaches to interpretating changes in HRQOL are available including two similar distribution approaches, e.g. Jacobson's Reliable Change Index [35,36] and Wyrwich's Standard Error of Measurement. [37,38]
The SF-6D is an example of a utility or preference-based measure of HRQoL. The primary use of such measures is to adjust life years saved by quality for use in economic evaluations and decision models. Preference-based health state scores or utilities do not have natural units. Since health is a function of both length of life and quality of life, the QALY (Quality-adjusted life year) has been developed in an attempt to combine the value of these attributes into a single index number. If utilities are multiplied by the amount of time spent in that particular health state then they become QALYs (and are measured in units of time). QALYs allow for varying times spent in different states by calculating an overall score for each patient. For the studies where the follow-up is one year (e.g. the two older adults cohorts) the mean change in utility scores over the one year can be directly interpreted as the MID for a QALY.
QALYs may have the potential to influence public policy and resource allocation decisions. Results from other preference based measures, such as the 15D and Health Utilities Index suggests a difference of 0.03 is considered the minimum clinically important difference for sample size calculations. Finally, as Drummond suggests, in the case of preference-based measures, if the ultimate objective is to influence resource allocation decisions, then it is the difference in cost-effectiveness (e.g. incremental cost per QALY) that is important, not the change in quality of life. Therefore changes in the measure alone may not be of interest without also considering the cost of bringing about such changes. [39]
Our findings are also limited in that a change in SF-6D score of 0.033 is important when the instrument is used for examining within-patient changes, but this does not necessarily mean that a difference of 0.033 will signify the MID when the instrument is used to discriminate between patients.
Despite the absence of a gold standard (criterion) measure, establishing the mean of any changes in a new measure like the SF-6D requires some sort of independent standard. The GRoC represents one credible alternative. Whilst we have not established with certainty a single best estimate of the MID for the SF-6D, our data suggest a plausible range within which the MID probably falls. This information will be useful in the interpreting SF-6D scores, both in individuals and in groups of patients participating in trials. It will also be useful in the planning of new trials, as sample size depends on the magnitude of the difference investigators consider important and are not willing to risk failing to detect. [40]
Summary and Conclusions
From the nine reviewed studies the MID for the SF-6D ranged from 0.010 to 0.048, weighted mean 0.033 (95% CI: 0.029 to 0.037). The corresponding SRMs ranged from 0.11 to 0.48, mean 0.30 and were mainly in the "small to moderate" range using Cohen's criteria, supporting the MID results. Using a half-standard deviation of change approach the mean effect size was 0.051 and ranged from 0.033 to 0.066. This suggests that the results obtained through the MID method are reasonable and generally of similar size to the effect size (SRM) estimates. It demonstrates that regardless of the method used, the actual cut-off point for a clinically important difference is going to be in the same neighbourhood, thereby making the particular method of approach less important. However, further empirical work is required to see whether or not these results hold true for other patient groups and populations.
Contributor Information
Stephen J Walters, Email: s.j.walters@sheffield.ac.uk.
John E Brazier, Email: j.e.brazier@sheffield.ac.uk.
References
- Sloan JA, Cella D, Frost M, Guyatt GH, Sprangers M, Symonds T, and the Clinical Significance Consensus Meeting Group Assessing clinical significance in measuring oncology patient quality of life: introduction to the symposium, content overview, and definition of terms. Mayo Clinic Proceedings. 2002;77:367–370. doi: 10.4065/77.4.367. [DOI] [PubMed] [Google Scholar]
- Guyatt GH, Osoba D, Wu AW, Wyrwich KW, Norman GR, and the Clinical Significance Consensus Meeting Group Methods to explain the clinical significance of health status measures. Mayo Clinic Proceedings. 2002;77:371–383. doi: 10.4065/77.4.371. [DOI] [PubMed] [Google Scholar]
- Cella D, Bullinger M, Scott C, Barofsky I, and the Clinical Significance Consensus Meeting Group Group vs. individual approaches to understanding the clinical significance of differences or changes in quality of life. Mayo Clinic Proceedings. 2002;77:384–392. doi: 10.4065/77.4.384. [DOI] [PubMed] [Google Scholar]
- Sloan JA, Aaronson N, Cappelleri JC, Fairclough DL, Varricchio C, Clinical Significance Consensus Meeting Group Assessing the clinical significance of single items relative to summated scores. Mayo Clinic Proceedings. 2002;77:479–487. [PubMed] [Google Scholar]
- Frost MH, Bonomi AE, Ferrans CE, Wong GY, Hays RD, and the Clinical Significance Consensus Meeting Group Patient, clinician, and population perspectives on determining the clinical significance of quality-of-life scores. Mayo Clinic Proceedings. 2002;77:488–494. [PubMed] [Google Scholar]
- Sprangers MA, Moinpour CM, Moynihan TJ, Patrick DL, Revicki DA, and the Clinical Significance Consensus Meeting Group Assessing meaningful change in quality of life over time: a users' guide for clinicians. Mayo Clinic Proceedings. 2002;77:561–571. doi: 10.4065/77.6.561. [DOI] [PubMed] [Google Scholar]
- Symonds T, Berzon R, Marquis P, Rummans TA, and the Clinical Significance Consensus Meeting Group The clinical significance of quality-of-life results: practical considerations for specific audiences. Mayo Clinic Proceedings. 2002;77:572–583. doi: 10.4065/77.6.572. [DOI] [PubMed] [Google Scholar]
- Fayers PM, Machin DM. Quality of Life: Assessment, Analysis & Interpretation. Chichester: Wiley; 2000. [Google Scholar]
- Jaeschke R, Singer J, Guyatt GH. Measurement of Health Status. Ascertaining the Minimal Clinically Important Difference. Controlled Clinical Trials. 1989;10:407–415. doi: 10.1016/0197-2456(89)90005-6. [DOI] [PubMed] [Google Scholar]
- Sloan J, Symonds T, Vargas-Chanes D, Fridley B. Practical Guidelines for Assessing the Clinical Significance of Health-Related Quality of Life Changes within Clinical Trials. Drug Information Journal. 2003;37:23–31. [Google Scholar]
- Juniper EF, Guyatt GH, Willan A, Griffith LE. Determining a minimal important change in a disease-specific Quality of Life Questionnaire. Journal of Clinical Epidemiology. 1994;47:81–87. doi: 10.1016/0895-4356(94)90036-1. [DOI] [PubMed] [Google Scholar]
- Norman GR, Sridhar FG, Guyatt GH, Walter SD. The Relation of Distribution- and Anchor-Based Approaches in Interpretation of Changes in Health Related Quality of Life. Medical Care. 2001;39:1039–1047. doi: 10.1097/00005650-200110000-00002. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioural Sciences. 2. New Jersey: Lawrence Earlbaum; 1988. [Google Scholar]
- Staquet MJ, Hays RD, Fayers PM. Quality of Life Assessment in Clinical Trials: Methods and Practice. Oxford University Press: Oxford; 1998. [Google Scholar]
- Kazis LE, Anderson JJ, Meenan RF. Effect Sizes for Interpreting Changes in Health Status. Medical Care. 1989;27:S178–S189. doi: 10.1097/00005650-198903001-00015. [DOI] [PubMed] [Google Scholar]
- Liang MH, Larson MG, Gullen KE, Schwartz JA. Comparative measurement efficiency and sensitivity of five health status instruments for arthritis research. Arthritis & Rheumatology. 1985;28:545–547. doi: 10.1002/art.1780280513. [DOI] [PubMed] [Google Scholar]
- Liang MH, Fossel AH, Larson MG. Comparisons of Five Health Status Instruments for Orthopaedic Evaluation. Medical Care. 1990;28:632–642. doi: 10.1097/00005650-199007000-00008. [DOI] [PubMed] [Google Scholar]
- Guyatt GH, Walter S, Norman G. Measuring change over time: assessing the usefulness of evaluative instruments. Journal of Chronic Disease. 1987;40:171–178. doi: 10.1016/0021-9681(87)90069-5. [DOI] [PubMed] [Google Scholar]
- Norman GR, Stratford P, Regehr G. Methodological Problems in the Retrospective Computation of Responsiveness to Change: The Lesson of Cronbach. J Clinical Epidemiology. 1997;50:869–879. doi: 10.1016/S0895-4356(97)00097-8. [DOI] [PubMed] [Google Scholar]
- Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Medical Care. 1992;30:473–483. [PubMed] [Google Scholar]
- Ware JE, Jr, Snow KK, Kosinski M, Gandek B. SF-36 Health Survey Manual and Interpretation Guide. Boston, MA: The Health Institute, New England Medical Centre; 1993. [Google Scholar]
- Angst F, Aeschlimann A, Stucki G. Smallest detectable and minimal clinically important differences of rehabilitation intervention with their implications for required sample sizes using WOMAC and SF-36 quality of life measurement instruments in patients with osteoarthritis of the lower extremities. Arthritis & Rheumatism. 2001;4:384–391. doi: 10.1002/1529-0131(200108)45:4<384::AID-ART352>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Hays RD, Morales LS. The RAND-36 measure of health-related quality of life. Annals of Medicine. 2001;33:350–357. doi: 10.3109/07853890109002089. [DOI] [PubMed] [Google Scholar]
- Brazier J, Usherwood T, Harper R, Thomas K. Deriving a Preference-based Single Index from the UK SF-36 Health Survey. J Clin Epidemiol. 1998;51:1115–1128. doi: 10.1016/S0895-4356(98)00103-6. [DOI] [PubMed] [Google Scholar]
- Brazier JE, Roberts JF, Deverill MD. The estimation of a preference based measure of health from the SF-36. Health Economics. 2002;21:271–292. doi: 10.1016/S0167-6296(01)00130-8. [DOI] [PubMed] [Google Scholar]
- Morrell CJ, Walters SJ, Dixon S, Collins KA, Brereton LML, Peters J, Brooker CGD. Cost-effectiveness of community leg ulcer clinics: randomised controlled trial. British Medical Journal. 1998;316:1487–1491. doi: 10.1136/bmj.316.7143.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters SJ, Munro JF, Brazier JE. Using the SF-36 with older adults: cross-sectional community based survey. Age & Ageing. 2001;30:337–343. doi: 10.1093/ageing/30.4.337. [DOI] [PubMed] [Google Scholar]
- Akehurst RL, Brazier JE, Mathers N, Healy C, Kaltenthaler E, Morgan AM, Platts M, Walters SJ. Health-related Quality of Life and Cost Impact of Irritable bowel Syndrome in a UK Primary Care Setting. Pharmacoeconomics. 2002;20:455–462. doi: 10.2165/00019053-200220070-00003. [DOI] [PubMed] [Google Scholar]
- Harper R, Brazier JE, Waterhouse JC, Walters SJ, Jones NMB, Howard P. Comparison of outcome measures for patients with chronic obstructive pulmonary disease (COPD) in an outpatient setting. Thorax. 1997;52:879–887. doi: 10.1136/thx.52.10.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazier JE, Harper R, Munro JF, Walters SJ, Snaith ML. Generic and condition-specific outcome measures for people with osteoarthritis of the knee. Rhemautology. 1999;38:870–877. doi: 10.1093/rheumatology/38.9.870. [DOI] [PubMed] [Google Scholar]
- Petitti D-B. Methods for Quantitative Synthesis in Medicine. Oxford, Oxford University Press; 1994. Meta-analysis, Decision Analysis, Cost-Effectiveness Analysis. [Google Scholar]
- Armitage P, Berry G, Matthews JNS. Statistical Methods in Medical Research. 4. Oxford: Blackwell; 2002. [Google Scholar]
- Efron B, Tibshirani RJ. An Introduction to the Bootstrap. New York: Chapman & Hall; 1993. [Google Scholar]
- Wyrwich KW, Metz SM, Babu AN, Kroenke K, Tierney WM, Wolinsky FD. The reliability of retrospective change assessments. Quality of Life Research. 2002;11:636. [Google Scholar]
- Jacobson NS, Truax P. Clinical Significance: A Statistical Approach to defining Meaningful Change in Psychotherapy Research. Journal of Consulting and Clinical Psychology. 1991;59:12–19. doi: 10.1037//0022-006X.59.1.12. [DOI] [PubMed] [Google Scholar]
- Fergason RJ, Robinson AB, Spaine M. Use of the Reliable Change Index to evaluate clinical significance in SF-36 outcomes. Quality of Life Research. 2002;11:509–516. doi: 10.1023/A:1016350431190. [DOI] [PubMed] [Google Scholar]
- Wyrwich KW, Nienaber NA, Tierney WM, Wolinsky FD. Linking Clinical Relevance and Statistical Significance in Evaluating Intra-Individual Changes in Health-Related Quality of Life. Medical Care. 1999;37:469–478. doi: 10.1097/00005650-199905000-00006. [DOI] [PubMed] [Google Scholar]
- Wyrwich KW, Tierney WM, Wolinsky FD. Using the standard error of measurement to identify important changes on the Asthma Quality of Life Questionnaire. Quality of Life Research. 2002;11:1–7. doi: 10.1023/A:1014485627744. [DOI] [PubMed] [Google Scholar]
- Drummond MF. Introducing economic and quality of life measures into clinical studies. Ann Med. 2001;33:344–349. doi: 10.3109/07853890109002088. [DOI] [PubMed] [Google Scholar]
- Walters SJ, Campbell MJ, Paisley S. Methods for determining sample sizes for studies involving quality of life measures: a tutorial. Health Services & Outcomes Research Methodology. 2001;2:83–99. doi: 10.1023/A:1020102612073. [DOI] [Google Scholar]


